Long non-coding RNA CASC7 is associated with the pathogenesis of heart failure via modulating the expression of miR-30c
- PMID: 32860492
- PMCID: PMC7576250
- DOI: 10.1111/jcmm.15764
Long non-coding RNA CASC7 is associated with the pathogenesis of heart failure via modulating the expression of miR-30c
Erratum in
-
.J Cell Mol Med. 2022 Apr;26(7):2133. doi: 10.1111/jcmm.17199. J Cell Mol Med. 2022. PMID: 35384288 Free PMC article. No abstract available.
Abstract
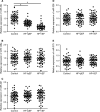
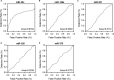
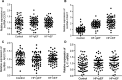
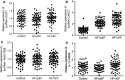
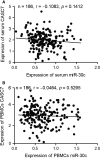
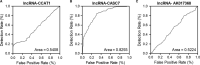
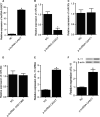
MiRNAs can be used as promising diagnostic biomarkers of heart failure, while lncRNAs act as competing endogenous RNAs of miRNAs. In this study, we collected peripheral blood monocytes from subjects with or without HF to explore the association between certain lncRNAs, miRNAs and HF. Heart failure patients with preserved or reduced ejection fraction were recruited for investigation. ROC analysis was carried out to evaluate the diagnostic values of certain miRNAs and lncRNAs in HF. Luciferase assays were used to study the regulatory relationship between above miRNAs and lncRNAs. LncRNA overexpression was used to explore the effect of certain miRNAs in H9C2 cells. Expression of miR-30c was significantly decreased in the plasma and peripheral blood monocytes of patients suffering from heart failure, especially in these with reduced ejection fraction. On the contrary, the expression of lncRNA-CASC7 was remarkably increased in the plasma and peripheral blood monocytes of patients suffering from heart failure. Both miR-30c and lncRNA-CASC7 expression showed a promising efficiency as diagnostic biomarkers of heart failure. Luciferase assays indicated that miR-30c played an inhibitory role in lncRNA-CASC7 and IL-11 mRNA expression. Moreover, the overexpression of lncRNA-CASC7 suppressed the expression of miR-30c while evidently increasing the expression of IL-11 mRNA and protein in H9C2 cells. This study clarified the relationship among miR-30c, lncRNA-CASC7 and IL-11 expression and the risk of heart failure and showed that lncRNA-CASC7 is potentially involved in the pathogenesis of HF via modulating the expression of miR-30c.
Keywords: CASC7; IL-11; cardiomyocyte; heart failure; lncRNA; miR-30c; miRNA.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

