Citicoline for treating people with acute ischemic stroke
- PMID: 32860632
- PMCID: PMC8406786
- DOI: 10.1002/14651858.CD013066.pub2
Citicoline for treating people with acute ischemic stroke
Abstract
Background: Stroke is one of the leading causes of long-lasting disability and mortality and its global burden has increased in the past two decades. Several therapies have been proposed for the recovery from, and treatment of, ischemic stroke. One of them is citicoline. This review assessed the benefits and harms of citicoline for treating patients with acute ischemic stroke.
Objectives: To assess the clinical benefits and harms of citicoline compared with placebo or any other control for treating people with acute ischemic stroke.
Search methods: We searched in the Cochrane Stroke Group Trials Register, CENTRAL, MEDLINE Ovid, Embase Ovid, LILACS until 29 January 2020. We searched the World Health Organization Clinical Trials Search Portal and ClinicalTrials.gov. Additionally, we also reviewed reference lists of the retrieved publications and review articles, and searched the websites of the US Food and Drug Administration (FDA) and European Medicines Agency (EMA).
Selection criteria: We included randomized controlled trials (RCTs) in any setting including participants with acute ischemic stroke. Trials were eligible for inclusion if they compared citicoline versus placebo or no intervention.
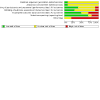
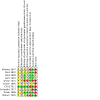
Data collection and analysis: We selected RCTs, assessed the risk of bias in seven domains, and extracted data by duplicate. Our primary outcomes of interest were all-cause mortality and the degree of disability or dependence in daily activities at 90 days. We estimated risk ratios (RRs) for dichotomous outcomes. We measured statistical heterogeneity using the I² statistic. We conducted our analyses using the fixed-effect and random-effects model meta-analyses. We assessed the overall quality of evidence for six pre-specified outcomes using the GRADE approach.
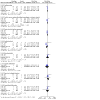
Main results: We identified 10 RCTs including 4281 participants. In all these trials, citicoline was given either orally, intravenously, or a combination of both compared with placebo or standard care therapy. Citicoline doses ranged between 500 mg and 2000 mg per day. We assessed all the included trials as having high risk of bias. Drug companies sponsored six trials. A pooled analysis of eight trials indicates there may be little or no difference in all-cause mortality comparing citicoline with placebo (17.3% versus 18.5%; RR 0.94, 95% CI 0.83 to 1.07; I² = 0%; low-quality evidence due to risk of bias). Four trials found no difference in the proportion of patients with disability or dependence in daily activities according to the Rankin scale comparing citicoline with placebo (21.72% versus 19.23%; RR 1.11, 95% CI 0.97 to 1.26; I² = 1%; low-quality evidence due to risk of bias). Meta-analysis of three trials indicates there may be little or no difference in serious cardiovascular adverse events comparing citicoline with placebo (8.83% versus 7.77%; RR 1.04, 95% CI 0.84 to 1.29; I² = 0%; low-quality evidence due to risk of bias). Overall, either serious or non-serious adverse events - central nervous system, gastrointestinal, musculoskeletal, etc. - were poorly reported and harms may have been underestimated. Four trials assessing functional recovery with the Barthel Index at a cut-off point of 95 points or more did not find differences comparing citicoline with placebo (32.78% versus 30.70%; RR 1.03, 95% CI 0.94 to 1.13; I² = 24%; low-quality evidence due to risk of bias). There were no differences in neurological function (National Institutes of Health Stroke Scale at a cut-off point of ≤ 1 points) comparing citicoline with placebo according to five trials (24.31% versus 22.44%; RR 1.08, 95% CI 0.96 to 1.21; I² = 27%, low-quality evidence due to risk of bias). A pre-planned Trial Sequential Analysis suggested that no more trials may be needed for the primary outcomes but no trial provided information on quality of life.
Authors' conclusions: This review assessed the clinical benefits and harms of citicoline compared with placebo or any other standard treatment for people with acute ischemic stroke. The findings of the review suggest there may be little to no difference between citicoline and its controls regarding all-cause mortality, disability or dependence in daily activities, severe adverse events, functional recovery and the assessment of the neurological function, based on low-certainty evidence. None of the included trials assessed quality of life and the safety profile of citicoline remains unknown. The available evidence is of low quality due to either limitations in the design or execution of the trials.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Arturo Martí‐Carvajal: none known. Claudia Valli: none known. Cristina Martí‐Amarista: none known. Ivan Solà: none known. Joan Martí‐Fàbregas: none known. Xavier Bonfill Cosp: none known.
Figures
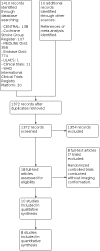


















Update of
- doi: 10.1002/14651858.CD013066
References
References to studies included in this review
Alviarez 2007 {published data only}
-
- Alvairez E, Gonzalez M. Effectiveness and tolerability of citicoline in acute ischemic stroke, randomized, double-blind study compared with placebo [Efectividad y tolerabilidad de la citicolina en el ictus isquémico agudo, estudio aleatorizado, doble ciego comparado con placebo]. Archivos Venezolanos de Farmacología y Terapéutica 2007;26(2):127-30. [ISSN 0798-0264]
Clark 1997 {published data only}
-
- Clark WM, Warach SJ, Pettigrew LC, Gammans RE, Sabounjian LA, Citicoline Stroke Study Group. A randomized dose-response trial of citicoline in acute ischemic stroke patients. Neurology 1997;49(3):671-8. [PMID: ] - PubMed
Clark 1999 {published data only}
-
- Clark WM, Williams BJ, Selzer KA, Zweifler RM, Sabounjian LA, Gammans RE. A randomized efficacy trial of citicoline in patients with acute ischemic stroke. Stroke 1999;30(12):2592-7. [PMID: ] - PubMed
Clark 2001 {published data only}
-
- Clark WM, Wechsler LR, Sabounjian LA, Schwiderski UE. A phase III randomized efficacy trial of 2000 mg citicoline in acute ischemic stroke patients. Neurology 2001;57(9):1595-602. [PMID: ] - PubMed
Ghosh 2015 {published data only}
-
- Ghosh S, Das SK, Nath T, Ghosh KC, Bhattacharyya R, Mondal GP. The effect of citicoline on stroke: a comparative study from the Eastern part of India. Neurology India 2015;63(5):697-701. [PMID: ] - PubMed
Guillen 1995 {published data only}
-
- Guillen F, Buendia C, Herrera J. CDP-choline in the treatment of acute ischaemic stroke. Journal of Neurology 1995;242 Suppl 2:S76.
ICTUS 2012 {published data only}
-
- Dávalos A, Alvarez-Sabín J, Castillo J, Díez-Tejedor E, Ferro J, Martínez-Vila E, International Citicoline Trial on acUte Stroke (ICTUS) trial investigators. Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial). Lancet 2012;380(9839):349-57. [PMID: ] - PubMed
Seifaddini 2017 {published data only}
-
- Seifaddini R, Moghadam AH, Iranmanesh F, Arvan H, Naghibzadeh-Tahami A. The effects of citicoline on cerebrovascular hemodynamic status in ischemic stroke patients. Journal of Kerman University of Medical Sciences 2017;24(6):480-6.
Tazaki 1988 {published data only}
-
- Tazaki Y, Sakai F, Otomo E, Kutsuzawa T, Kameyama M, Omae T, et al. Treatment of acute cerebral infarction with a choline precursor in a multicenter double-blind placebo-controlled study. Stroke 1988;19(2):211-6. [PMID: ] - PubMed
Warach 2000 {published data only}
-
- Warach S, Pettigrew LC, Dashe JF, Pullicino P, Lefkowitz DM, Sabounjian L, Citicoline 010 Investigators. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Annals of Neurology 2000;48(5):713-22. [PMID: ] - PubMed
References to studies excluded from this review
Dereux 1987 {published data only}
-
- Dereux J-F, Gallois P. Comparison of the results with corticotrophin and citicoline in the initial stage of cerebral infarction. Gazette Medicale 1987;94(4):82-5. [CN-00324812]
Djoenady 1981 {published data only}
-
- Djoenaidi W, Susilo H, Hakim AA, Batubara A, Koentioro EA. A double-blind controlled trial of cytidine-diphosphate-choline (CDPcholine) on thrombotic stroke patients. 12th World Congress of Neurology, Kyoto (Japan), September (Abstract 869) 1981.
ECCS‐ AIS 2012 {published data only}
-
- Goel D. ECCT-HIS. Edaravone-Citicoline comparative trial in head injury and stroke. Available at www.strokecenter.org/trials/clinicalstudies/edaravone-citicoline-compara....
-
- Mittal M, Goel D, Bansal KK, Puri P. Edaravone - citicoline comparative study in acute ischemic stroke (ECCS-AIS). Journal of the Association of Physicians of India 2012;60(11):36-8. [PMID: ] - PubMed
Garcia Pastor 2004 {published data only}
-
- Garcia Pastor A, Diaz Otero F, Gil Nunez AC, Villanueva Osorio JA. Tolerability and efficacy of the combination of piracetam and citicoline in acute ischemic stroke. A randomized comparative open study. Stroke 2004;35(6):e286.
Goas 1980 {published data only}
-
- Goas JY, Bastard J, Missoum A. Results after 90 days of stroke treatment with CDP-choline concerning a double-blind test [Bilan a 90 jours du traitement des accidents vasculaires cerebraux par la CDP-choline. A propos d' un essai en double insu]. International Symposium. Brain Suffering and Precursor of Phospholipids. Paris 1980, January 18;109-21. 1980:123-8.
Melnikova 2011 {published data only}
-
- Melnikova EV, Shmonin AA, Kulagin PA, Stukova LN, Mischenko KA. Neuroprotection effectiveness depends on the size and location of the ischemic stroke focus. Cerebrovascular Diseases 2011;31 Suppl 2:132.
Panteleienko 2009 {published data only}
-
- Panteleienko L, Sokolova L. Patients treated with citicoline in acute phase of ischemic stroke report better quality of life. European Journal of Neurology 2009;16(S3):412.
References to studies awaiting assessment
Hassan 2019 {published data only}
-
- Hassan A, Hassoun HK, Sameer A, Hadi NR, Allebban Z. Effect of citicoline on cerebral hemodynamics and vasoreactivity using transcranial doppler in acute ischemic stroke patients: clinical trial. International Journal of Pharmaceutical Research 2019;11(2):262-9. [DOI: 10.31838/ijpr/2019.11.02.045] - DOI
References to ongoing studies
CTRI/2018/02/011900 {published data only}
-
- Agarwal A, Vishnu VY, Bhatia R, Goyal V, Singh MB, Joseph L, et al. [Abstract 431] Citicoline in acute ischemic stroke - a randomized control trial. International Journal of Stroke 2018;13(2S):104.
IRCT201601289014N90 {published data only}
-
- Ongoing study. Expected recruitment start date: 20 February 2016Expected recruitment end date: 18 February 2017. Contact author for more information.
Additional references
Allen 2018
Altman 1998
Astrup 1981
-
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke 1981;12(6):723-5. [PMID: ] - PubMed
Astrup 1982
-
- Astrup J. Energy-requiring cell functions in the ischemic brain. Their critical supply and possible inhibition in protective therapy. Journal of Neurosurgery 1982;56(4):482-97. [PMID: ] - PubMed
Atkins 2004
Avarez‐Sabín 2013
Barnes 1998
-
- Barnes DE, Bero LA. Why review articles on the health effects of passive smoking reach different conclusions. JAMA 1998;279(19):1566-70. [PMID: ] - PubMed
Bath 2011
-
- Bath P, Hogg C, Tracy M, Pocock S. Calculation of numbers-needed-to-treat in parallel group trials assessing ordinal outcomes: case examples from acute stroke and stroke prevention. International Journal of Stroke 2011;6(6):472-9. [PMID: ] - PubMed
Bath 2012
-
- Bath PM, Lees KR, Schellinger PD, Altman H, Bland M, Hogg C, et al. Statistical analysis of the primary outcome in acute stroke trials. Stroke 2012;43(4):1171-8. [PMID: ] - PubMed
Bland 2000
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial Sequential Analysis reveals insufficient information size and potentially false positive results in many meta-analyses. Journal of Clinical Epidemiology 2008;61(8):763-9. [PMID: ] - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive: Trial Sequential Analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. International Journal of Epidemiology 2009;38(1):287-98. [PMID: ] - PubMed
Carroll 2011
-
- Carroll DG. Functional evaluation: the Barthel index. www.strokecenter.org/wp-content/uploads/2011/08/barthel_reprint.pdf (accessed 25 May 2017).
Cassella 2017
-
- Cassella CR, Jagoda A. Ischemic stroke: advances in diagnosis and management. Emergency Medicine Clinics of North America 2017;35(4):911-30. [PMID: ] - PubMed
Chaimani 2014
-
- Chaimani A, Mavridis D, Salanti G. A hands-on practical tutorial on performing meta-analysis with Stata. Evidence-based Mental Health 2014;17(4):111-6. [PMID: ] - PubMed
Chan 2008
Chen 2016
Clark 2009
-
- Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opinion on Pharmacotherapy 2009;10(5):839-46. [PMID: 19351232 ] - PubMed
CONSORT 2010
-
- CONSORT. Consolidated Standards of Reporting Trials. www.consort-statement.org/ (accessed 8 July 2020).
CTU 2011
-
- Copenhagen Trial Unit. TSA - Trial Sequential Analysis. ctu.dk/tsa/ (accessed 25 May 2017).
Dávalos 2002
-
- Dávalos A, Castillo J, Alvarez-Sabin J, Secades JJ, Mercadal J, Lopez S, et al. Oral citicoline in acute ischemic stroke: an individual patient data pooling analysis of clinical trials. Stroke 2002;33(12):2850-7. [PMID: ] - PubMed
Davies 1998
Deeks 2017
-
- Deeks JJ, Higgins JPT, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017.The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Delorme 2016
-
- Delorme P, Micheaux PL, Liquet B, Riou J. Type-II generalized family-wise error rate formulas with application to sample size determination. Statistics in Medicine 2016;35(16):2687-714. [PMID: ] - PubMed
Dienes 2008
-
- Dienes Z. Understanding Psychology as a Science: An Introduction to Scientific and Statistical Inference. 1st edition. London: Palgrave Macmillan, 2008. [9780230542303]
Dienes 2014
Dienes 2018
FDA 2019
-
- US Food and Drug Administration. www.fda.gov/inspections-compliance-enforcement-and-criminal-investigatio... 2019.
Friedrich 2011
-
- Friedrich JO, Adhikari NK, Beyene J. Ratio of means for analyzing continuous outcomes in meta-analysis performed as well as mean difference methods. Journal of Clinical Epidemiology 2011;64(5):556-64. [PMID: ] - PubMed
Furukawa 1999
-
- Furukawa TA. From effect size into number needed to treat. Lancet 1999;353(9165):1680. [PMID: ] - PubMed
Furukawa 2011
Gamble 2005
-
- Gamble C, Hollis S. Uncertainty method improved on best-worst case analysis in a binary meta-analysis. Journal of Clinical Epidemiology 2005;58(6):579-88. [PMID: ] - PubMed
Goodman 1999
-
- Goodman SN. Toward evidence-based medical statistics. 2: The Bayes factor. Annals of Internal Medicine 1999;130(12):1005-13. [PMID: ] - PubMed
Goodman 2005
-
- Goodman SN. Introduction to Bayesian methods I: measuring the strength of evidence. Clinical Trials 2005;2(4):282-90. [PMID: ] - PubMed
GRADEpro GDT 2015 [Computer program]
-
- GRADEpro GDT. Version accessed 29 May 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Grieb 2014
Hankey 2017
-
- Hankey GJ. Stroke. Lancet 2017;389(10069):641-54. [PMID: ] - PubMed
Harrison 2013
Higgins 2003
Higgins 2011a
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from handbook.cochrane.org.
Higgins 2019
-
- Higgins JPT, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:143-176.
Hillis 2015
Hoffmann 2014
-
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [PMID: ] - PubMed
Hoffmann 2017
-
- Hoffmann TC, Oxman AD, Ioannidis JP, Moher D, Lasserson TJ, Tovey DI, et al. Enhancing the usability of systematic reviews by improving the consideration and description of interventions. BMJ 2017;357:j2998. [PMID: ] - PubMed
Hollis 1999
Imberger 2015
-
- Imberger G, Gluud C, Boylan J, Wetterslev J. Systematic reviews of anesthesiologic interventions reported as statistically significant: problems with power, precision, and type 1 error protection. Anesthesia and Analgesia 2015;121(6):1611-22. [PMID: ] - PubMed
Imberger 2016
IntHout 2016
Ioannidis 2009
-
- Ioannidis JP. Adverse events in randomized trials: neglected, restricted, distorted, and silenced. Archives of Internal Medicine 2009;169(19):1737-9. [PMID: ] - PubMed
Ioannidis 2010
-
- Ioannidis JP. Meta-research: The art of getting it wrong. Research synthesis methods 2010;1(3-4):169-84. [PMID: ] - PubMed
Ioannidis 2014
Jakobsen 2014
Johnston 2016
Lan 1983
-
- Lan GK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70(3):659-63. [DOI: 10.1093/biomet/70.3.659] - DOI
Laupacis 1988
-
- Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. New England Journal of Medicine 1988;318(26):1728-33. [PMID: ] - PubMed
Lee 2010
-
- Lee M, Towfighi A, Saver JL. Choline precursors in acute and subacute ischemic and hemorrhagic stroke: an updated meta-analysis of randomized controlled trials. Stroke 2010;41 (4):e263 (Abstract 33).
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lineberry 2016
-
- Lineberry N, Berlin JA, Mansi B, Glasser S, Berkwits M, Klem C, et al. Recommendations to improve adverse event reporting in clinical trial publications: a joint pharmaceutical industry/journal editor perspective. BMJ 2016;355:i5078. [PMID: ] - PubMed
Liu 2011
-
- Liu L, Shi Z, Yan Q, Guo J. Cytidine diphosphate choline for acute stroke: a meta-analysis. Chinese Journal of Evidence-based Medicine 2011;11(4):404-12.
Lundh 2017
Mahoney 1965
-
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Maryland State Medical Journal 1965;14:61-5. [PMID: ] - PubMed
Manning 2014
-
- Manning NW, Campbell BC, Oxley TJ, Chapot R. Acute ischemic stroke: time, penumbra, and reperfusion. Stroke 2014;45(2):640-4. [PMID: ] - PubMed
Martynov 2015
Mascha 2018
-
- Mascha EJ, Vetter TR. Significance, errors, power, and sample size: The blocking and tackling of statistics. Anesthesia and Analgesia 2018;126(2):691-8. [PMID: ] - PubMed
McArthur 2014
-
- McArthur KS. Improving efficiency in stroke trials: an exploration of methods to improve the use of the modified Rankin Scale in acute stroke trials. theses.gla.ac.uk/5350 2014:1-261.
Mhaskar 2012
NIHSS
-
- NIH Stroke Scale/Score (NIHSS). www.mdcalc.com/nih-stroke-scale-score-nihss (accessed 6 April 2018).
Overgaard 2014
-
- Overgaard K. The effects of citicoline on acute ischemic stroke: a review. Journal of Stroke and Cerebrovascular Diseases 2014;23(7):1764-9. [PMID: ] - PubMed
Patel 2017
-
- Patel RA, McMullen PW. Neuroprotection in the treatment of acute ischemic stroke. Progress in Cardiovascular Diseases 2017;59(6):542-8. [PMID: ] - PubMed
Peters 2008
-
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991-6. [PMID: ] - PubMed
Pitrou 2009
-
- Pitrou I, Boutron I, Ahmad N, Ravaud P. Reporting of safety results in published reports of randomized controlled trials. Archives of Internal Medicine 2009;169(19):1756-61. [PMID: ] - PubMed
Porta 2014
-
- Porta M. A Dictionary of Epidemiology. 6th edition. New York: Oxford University Press, 2014. [ISBN 978-0-19-997672-0]
Portegies 2016
-
- Portegies ML, Koudstaal PJ, Ikram MA. Cerebrovascular disease. In: Aminoff MJ, Boller F, Swaa DF, editors(s). Handbook of Clinical Neurology. Vol. 138. Radarweg, Amsterdam, Netherlands: Elsevier, 2016:239-61. [ISBN: 978-0-12-802973-2] [PMID: ] - PubMed
Rajah 2017
-
- Rajah GB, Ding Y. Experimental neuroprotection in ischemic stroke: a concise review. Neurosurgical Focus 2017;42(4):E2. [PMID: ] - PubMed
Review Manager 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
-
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [PMID: ] - PubMed
Sackett 1996
-
- Sackett DL, Deeks J, Altman DG. Down with odds ratios! Evidence-based Medicine 1996;1:164-6.
Saver 2002
-
- Saver JL, Wilterdink J. Choline precursors in acute and subacute human stroke: a meta-analysis. Stroke 2002;33(1):353.
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al In: Higgins JP, Green S, editor(s) Cochrane Handbook for Systematic Reviews of Interventions Version 510 (updated March 2011) The Cochrane Collaboration, 2011. Chapter 11: Interpreting results and drawing conclusions. Available from handbook.cochrane.org.
Scott 1997
-
- Scott SC, Goldberg MS, Mayo NE. Statistical assessment of ordinal outcomes in comparative studies. Journal of Clinical Epidemiology 1997;50(1):45-55. [PMID: ] - PubMed
Secades 2016
-
- Secades JJ, Alvarez-Sabin J, Castillo J, Diez-Tejedor E, Martinez-Vila E, Rios J, et al. Citicoline for acute ischemic stroke: a systematic review and formal meta-analysis of randomized, double-blind, and placebo-controlled trials. Journal of Stroke and Cerebrovascular Diseases 2016;25(8):1984-96. [PMID: ] - PubMed
Senn 2007
-
- Senn S, Bretz F. Power and sample size when multiple endpoints are considered. Pharmaceutical Satistics 2007;6(3):161-70. [PMID: ] - PubMed
Shi 2016
-
- Shi PY, Zhou XC, Yin XX, Xu LL, Zhang XM, Bai HY. Early application of citicoline in the treatment of acute stroke: A meta-analysis of randomized controlled trials. Journal of Huazhong University of Science and Technology. Medical Sciences 2016;36(2):270-7. [MEDLINE: 27072975 ] - PubMed
Sormani 2017
-
- Sormani MP. The most frequently asked question to a statistician: the sample size. Multiple Sclerosis 2017;23(5):644-6. [PMID: ] - PubMed
SPIRIT 2013
-
- SPIRIT. Standard Protocol Items: Recommendations for Interventional Trials. www.spirit-statement.org/ (accessed 8 July 2020).
STATA
-
- StataCorp LLC. Stata 14. www.stata.com.
Sterne 2001
-
- Sterne JA, Bradburn MJ, Egger M. Meta-analysis in StataTM. In: Egger M, Smith DS, and Altman DG, editors(s). Systematic Reviews in Health Care: Meta-analysis in Context. 2nd edition. London (UK): BMJ Books, 2001:347-69. [978-0-7279-1488-0]
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [PMID: ] - PubMed
Thorlund 2010
Thorlund 2011a
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 6 March 2017).
Thorlund 2011b
van der Laan 2004
-
- Laan MJ, Dudoit S, Pollard KS. Augmentation procedures for control of the generalized family-wise error rate and tail probabilities for the proportion of false positives. Statistical Applications in Genetics and Molecular Biology 2004;3:Article 15. [PMID: ] - PubMed
van Swieten 1988
-
- Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19(5):604-7. [PMID: ] - PubMed
Wang 2016
-
- Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–544. [PMID: 27733281 ] - PMC - PubMed
Wang 2017
Wardlaw 2014
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. [PMID: ] - PubMed
Wetterslev 2009
Wetterslev 2017
Yan 2017
-
- Yan LL, Li C, Chen J, Luo R, Bettger J, Zhu Y, et al. Stroke. In: Prabhakaran D, Anand S, Gaziano TA, Mbanya JC, Wu Y, Nugent R, editors(s). Disease Control Priorities (openknowledge.worldbank.org/handle/10986/28875). 3rd edition. Vol. 5. Washington (DC): World Bank, 2017:157-76. [ISBN (electronic): 978-1-4648-0520-2]
Yang 2019
-
- Yang F, Wittes J, Pitt B. Beware of on-treatment safety analyses. Clinical Trials 2019;16(1):63-70. [PMID: ] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

