Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer
- PMID: 32860704
- PMCID: PMC7953739
- DOI: 10.1084/jem.20190354
Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer
Abstract
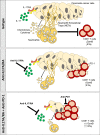
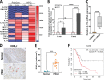
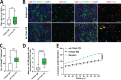
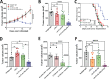
Pancreatic ductal adenocarcinoma (PDAC) remains a lethal malignancy with an immunosuppressive microenvironment that is resistant to most therapies. IL17 is involved in pancreatic tumorigenesis, but its role in invasive PDAC is undetermined. We hypothesized that IL17 triggers and sustains PDAC immunosuppression. We inhibited IL17/IL17RA signaling using pharmacological and genetic strategies alongside mass cytometry and multiplex immunofluorescence techniques. We uncovered that IL17 recruits neutrophils, triggers neutrophil extracellular traps (NETs), and excludes cytotoxic CD8 T cells from tumors. Additionally, IL17 blockade increases immune checkpoint blockade (PD-1, CTLA4) sensitivity. Inhibition of neutrophils or Padi4-dependent NETosis phenocopies IL17 neutralization. NMR spectroscopy revealed changes in tumor lactate as a potential early biomarker for IL17/PD-1 combination efficacy. Higher expression of IL17 and PADI4 in human PDAC corresponds with poorer prognosis, and the serum of patients with PDAC has higher potential for NETosis. Clinical studies with IL17 and checkpoint blockade represent a novel combinatorial therapy with potential efficacy for this lethal disease.
© 2020 Zhang et al.
Conflict of interest statement
Disclosure: Dr. Maitra reports Thrive Earlier Detection has licensed an invention from Johns Hopkins University in which Dr. Maitra is listed as an inventor. The focus of the license is on pancreatic cancer early detection. In addition, Dr. Maitra receives royalties from Cosmos Wisdom Biotechnology Ltd on an invention related to pancreatic cancer early detection, licensed from MD Anderson Cancer Center. Dr. Banerjee is a paid consultant with Minneamrita Therapeutics; this is managed by the University of Miami. No other disclosures were reported.
Figures







References
-
- Barnes, B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., et al. . 2020. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 217. e20200652. 10.1084/jem.20200652 - DOI - PMC - PubMed
-
- Bayne, L.J., Beatty G.L., Jhala N., Clark C.E., Rhim A.D., Stanger B.Z., and Vonderheide R.H.. 2012. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 21:822–835. 10.1016/j.ccr.2012.04.025 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

