Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients
- PMID: 32861333
- PMCID: PMC7368917
- DOI: 10.1016/j.mayocp.2020.06.028
Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients
Abstract
Objective: To provide an update on key safety metrics after transfusion of convalescent plasma in hospitalized coronavirus 2019 (COVID-19) patients, having previously demonstrated safety in 5000 hospitalized patients.
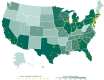
Patients and methods: From April 3 to June 2, 2020, the US Food and Drug Administration Expanded Access Program for COVID-19 convalescent plasma transfused a convenience sample of 20,000 hospitalized patients with COVID-19 convalescent plasma.
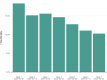
Results: The incidence of all serious adverse events was low; these included transfusion reactions (n=78; <1%), thromboembolic or thrombotic events (n=113; <1%), and cardiac events (n=677, ~3%). Notably, the vast majority of the thromboembolic or thrombotic events (n=75) and cardiac events (n=597) were judged to be unrelated to the plasma transfusion per se. The 7-day mortality rate was 13.0% (12.5%, 13.4%), and was higher among more critically ill patients relative to less ill counterparts, including patients admitted to the intensive care unit versus those not admitted (15.6 vs 9.3%), mechanically ventilated versus not ventilated (18.3% vs 9.9%), and with septic shock or multiple organ dysfunction/failure versus those without dysfunction/failure (21.7% vs 11.5%).
Conclusion: These updated data provide robust evidence that transfusion of convalescent plasma is safe in hospitalized patients with COVID-19, and support the notion that earlier administration of plasma within the clinical course of COVID-19 is more likely to reduce mortality.
Copyright © 2020. Published by Elsevier Inc.
Figures
Comment in
-
Limitations of Safety Update on Convalescent Plasma Transfusion in COVID-19 Patients.Mayo Clin Proc. 2020 Dec;95(12):2801-2802. doi: 10.1016/j.mayocp.2020.09.033. Epub 2020 Oct 1. Mayo Clin Proc. 2020. PMID: 33276848 Free PMC article. No abstract available.
-
In Reply - Limitations of Safety Update on Convalescent Plasma Transfusion in COVID-19 Patients.Mayo Clin Proc. 2020 Dec;95(12):2802-2803. doi: 10.1016/j.mayocp.2020.09.032. Epub 2020 Oct 1. Mayo Clin Proc. 2020. PMID: 33276849 Free PMC article. No abstract available.
-
In Reply - Micro-Thrombosis, Perfusion Defects, and Worsening Oxygenation in COVID-19 Patients: A Word of Caution on the Use of Convalescent Plasma.Mayo Clin Proc. 2021 Jan;96(1):259-261. doi: 10.1016/j.mayocp.2020.10.036. Epub 2020 Nov 3. Mayo Clin Proc. 2021. PMID: 33413824 Free PMC article. No abstract available.
-
Micro-Thrombosis, Perfusion Defects, and Worsening Oxygenation in COVID-19 Patients: A Word of Caution on the Use of Convalescent Plasma.Mayo Clin Proc. 2021 Jan;96(1):259. doi: 10.1016/j.mayocp.2020.10.035. Epub 2020 Nov 3. Mayo Clin Proc. 2021. PMID: 33413825 Free PMC article. No abstract available.
-
COVID-19 convalescent plasma therapy: hit fast, hit hard!Vox Sang. 2021 Oct;116(9):935-942. doi: 10.1111/vox.13091. Epub 2021 Apr 1. Vox Sang. 2021. PMID: 33794556 Free PMC article. No abstract available.
References
-
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed August 4, 2020.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

