A probabilistic atlas of locus coeruleus pathways to transentorhinal cortex for connectome imaging in Alzheimer's disease
- PMID: 32861791
- PMCID: PMC7797167
- DOI: 10.1016/j.neuroimage.2020.117301
A probabilistic atlas of locus coeruleus pathways to transentorhinal cortex for connectome imaging in Alzheimer's disease
Abstract
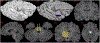
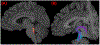
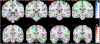
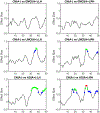
According to the latest Braak staging of Alzheimer's disease (AD), tau pathology occurs earliest in the brain in the locus coeruleus (LC) of the brainstem, then propagates to the transentorhinal cortex (TEC), and later to other neocortical regions. Recent animal and in vivo human brain imaging research also support the trans-axonal propagation of tau pathology. In addition, neurochemical studies link norepinephrine to behavioral symptoms in AD. It is thus critical to examine the integrity of the LC-TEC pathway in studying the early development of the disease, but there has been limited work in this direction. By leveraging the high-resolution and multi-shell diffusion MRI data from the Human Connectome Project (HCP), in this work we develop a novel method for the reconstruction of the LC-TEC pathway in a cohort of 40 HCP subjects carefully selected based on rigorous quality control of the residual distortion artifacts in the brainstem. A probabilistic atlas of the LC-TEC pathway of both hemispheres is then developed in the MNI152 space and distributed publicly on the NITRC website. To apply our atlas on clinical imaging data, we develop an automated approach to calculate the medial core of the LC-TEC pathway for localized analysis of connectivity changes. In a cohort of 138 subjects from the Alzheimer's Disease Neuroimaging Initiative (ADNI), we demonstrate the detection of the decreased fiber integrity in the LC-TEC pathways with increasing disease severity.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

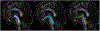

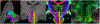

References
-
- Benarroch EE, 2009. The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology 73, 1699–1704. - PubMed
-
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc 57, 289–300.
-
- Betts MJ, Cardenas-Blanco A, Kanowski M, Jessen F, Düzel E, 2017. In vivo MRI assessment of the human locus coeruleus along its rostrocaudal extent in young and older adults. Neuroimage 163, 150–159. - PubMed
Publication types
MeSH terms
Grants and funding
- P41 EB015922/EB/NIBIB NIH HHS/United States
- U01 AG051218/AG/NIA NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- RF1 AG064584/AG/NIA NIH HHS/United States
- R21 AG064776/AG/NIA NIH HHS/United States
- RF1 AG056573/AG/NIA NIH HHS/United States
- CIHR/Canada
- P30 AG066530/AG/NIA NIH HHS/United States
- U54 MH091657/MH/NIMH NIH HHS/United States
- R01 AG062007/AG/NIA NIH HHS/United States
- UH3 NS100614/NS/NINDS NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- U01 EY025864/EY/NEI NIH HHS/United States
- R01 AG025340/AG/NIA NIH HHS/United States
- R01 EB022744/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

