Tumor neoantigen heterogeneity impacts bystander immune inhibition of pancreatic cancer growth
- PMID: 32862105
- PMCID: PMC7475277
- DOI: 10.1016/j.tranon.2020.100856
Tumor neoantigen heterogeneity impacts bystander immune inhibition of pancreatic cancer growth
Abstract
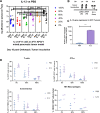
The immunogenic clonal-fraction threshold in heterogeneous solid-tumor required to induce effective bystander-killing of non-immunogenic subclones is unknown. Pancreatic cancer poses crucial challenges for immune therapeutic interventions due to low mutational-burden and consequent lack of neoantigens. Here, we designed a model to incorporate artificial-neoantigens into genes-of -interest in cancer-cells and to test their potential to actuate bystander-killing. By precisely controlling a neoantigen's abundance in the tumor, we studied the impact of neoantigen frequency on immune-response and immune-escape. Our results showed single, strong, widely-expressed neoantigen could lead to robust antitumor response when over 80% of cancer cells express the neoantigen. Further, immunological assays demonstrated T-cell responses against non-target self-antigen on KRAS-oncoprotein, when we inoculated animals with a high frequency of tumor-cells expressing test-neoantigen. Using nanoparticle-based gene-therapy, we successfully altered tumor-microenvironment by perturbing interleukin-12 and interleukin-10 gene-expression. The subsequent microenvironment-remodeling reduced the neoantigen frequency threshold at which bioluminescent signal intensity for tumor-burden decreased 1.5-log-fold, marking robust tumor-growth inhibition, from 83% to 29%. Our results thus suggest bystander killing is inefficient in immunologically-cold tumors like pancreatic-cancer and requires high neoantigen abundance. However, bystander killing mediated antitumor response can be rescued by adjuvant-immune therapy.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Antonarakis E.S. Immune responses and clinical outcomes in STAND, a randomized phase 2 study evaluating optimal sequencing of sipuleucel-T (sip-T) and androgen deprivation therapy (ADT) in biochemically-recurrent prostate cancer (BRPC) after local therapy failure. J. Clin. Oncol. 2015;33:5030.
-
- Hardwick N., Chain B. Epitope spreading contributes to effective immunotherapy in metastatic melanoma patients. Immunotherapy. 2011;3:731–733. - PubMed
-
- Taylor C. Augmented HER-2–specific immunity during treatment with trastuzumab and chemotherapy. Clin. Cancer Res. 2007;13:5133–5143. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

