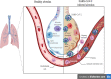
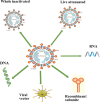
Overview of the current promising approaches for the development of an effective severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine
- PMID: 32862110
- PMCID: PMC7444935
- DOI: 10.1016/j.intimp.2020.106928
Overview of the current promising approaches for the development of an effective severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine
Abstract
Coronavirus disease 2019 (COVID-19) is a pandemic infectious disease caused by the novel coronavirus called SARS-CoV-2. There is a gap in our understanding regarding the immunopathogenesis of COVID-19. However, many clinical trials are underway across the world for screening effective drugs against COVID-19. Nevertheless, currently, no proven effective therapies for this virus exists. The vaccines are deemed as a significant part of disease prevention for emerging viral diseases, since, in several cases, other therapeutic choices are limited or non-existent, or that diseases result in such an accelerated clinical worsening that the efficacy of treatments is restricted. Therefore, effective vaccines against COVID-19 are urgently required to overcome the tremendous burden of mortality and morbidity correlated with SARS-CoV-2. In this review, we will describe the latest evidence regarding outstanding vaccine approaches and the challenges for vaccine production.
Keywords: COVID-19; Immunopathogenesis; SARS-CoV-2; Vaccines.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Nothing declared.
Figures
Similar articles
-
A systematic review of SARS-CoV-2 vaccine candidates.Signal Transduct Target Ther. 2020 Oct 13;5(1):237. doi: 10.1038/s41392-020-00352-y. Signal Transduct Target Ther. 2020. PMID: 33051445 Free PMC article.
-
SARS-CoV-2: A New Song Recalls an Old Melody.Cell Host Microbe. 2020 May 13;27(5):692-694. doi: 10.1016/j.chom.2020.04.019. Cell Host Microbe. 2020. PMID: 32407706 Free PMC article. Review.
-
The 2020 Pandemic: Current SARS-CoV-2 Vaccine Development.Front Immunol. 2020 Aug 19;11:1880. doi: 10.3389/fimmu.2020.01880. eCollection 2020. Front Immunol. 2020. PMID: 32973779 Free PMC article. Review.
-
Prospect of SARS-CoV-2 spike protein: Potential role in vaccine and therapeutic development.Virus Res. 2020 Oct 15;288:198141. doi: 10.1016/j.virusres.2020.198141. Epub 2020 Aug 23. Virus Res. 2020. PMID: 32846196 Free PMC article. Review.
-
COVID-19 Vaccine: A comprehensive status report.Virus Res. 2020 Oct 15;288:198114. doi: 10.1016/j.virusres.2020.198114. Epub 2020 Aug 13. Virus Res. 2020. PMID: 32800805 Free PMC article. Review.
Cited by
-
Effect of ambient temperature on respiratory tract cells exposed to SARS-CoV-2 viral mimicking nanospheres-An experimental study.Biointerphases. 2021 Jan 28;16(1):011006. doi: 10.1116/6.0000743. Biointerphases. 2021. PMID: 33706521 Free PMC article.
-
Identifying SARS-CoV-2 Lineage Mutation Hallmarks and Correlating Them With Clinical Outcomes in Egypt: A Pilot Study.Front Mol Biosci. 2022 Mar 8;9:817735. doi: 10.3389/fmolb.2022.817735. eCollection 2022. Front Mol Biosci. 2022. PMID: 35350713 Free PMC article.
-
The emerging role of microRNAs in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.Int Immunopharmacol. 2021 Jan;90:107204. doi: 10.1016/j.intimp.2020.107204. Epub 2020 Nov 13. Int Immunopharmacol. 2021. PMID: 33221169 Free PMC article. Review.
-
An overview of current drugs and prophylactic vaccines for coronavirus disease 2019 (COVID-19).Cell Mol Biol Lett. 2022 May 13;27(1):38. doi: 10.1186/s11658-022-00339-3. Cell Mol Biol Lett. 2022. PMID: 35562685 Free PMC article. Review.
-
Inhibition of SARS-CoV-2 pseudovirus invasion by ACE2 protecting and Spike neutralizing peptides: An alternative approach to COVID19 prevention and therapy.Int J Biol Sci. 2021 Jul 13;17(11):2957-2969. doi: 10.7150/ijbs.61476. eCollection 2021. Int J Biol Sci. 2021. PMID: 34345219 Free PMC article.
References
-
- T.W.H. Organization, Coronavirus disease (COVID-19) pandemic, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
-
- Parvin F., Islam S., Urmy Z., Ahmed S. The symptoms, contagious process, prevention and post treatment of COVID-19. Europ. J. Physiotherapy Rehabilit. Stud. 2020
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous