Effect of Low-Pathogenic Human Coronavirus-Specific Antibodies on SARS-CoV-2
- PMID: 32863133
- PMCID: PMC7418642
- DOI: 10.1016/j.it.2020.08.003
Effect of Low-Pathogenic Human Coronavirus-Specific Antibodies on SARS-CoV-2
Figures
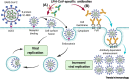
Comment on
-
Does Cross-neutralization of SARS-CoV-2 Only Relate to High Pathogenic Coronaviruses?Trends Immunol. 2020 Oct;41(10):851-853. doi: 10.1016/j.it.2020.08.002. Epub 2020 Aug 8. Trends Immunol. 2020. PMID: 32863136 Free PMC article. No abstract available.
References
-
- Pinto D. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

