Fyn specifically Regulates the activity of red cell glucose-6-phosphate-dehydrogenase
- PMID: 32863204
- PMCID: PMC7387845
- DOI: 10.1016/j.redox.2020.101639
Fyn specifically Regulates the activity of red cell glucose-6-phosphate-dehydrogenase
Abstract
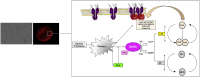
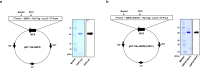
Fyn is a tyrosine kinase belonging to the Src family (Src-Family-Kinase, SFK), ubiquitously expressed. Previously, we report that Fyn is important in stress erythropoiesis. Here, we show that in red cells Fyn specifically stimulates G6PD activity, resulting in a 3-fold increase enzyme catalytic activity (kcat) by phosphorylating tyrosine (Tyr)-401. We found Tyr-401 on G6PD as functional target of Fyn in normal human red blood cells (RBC), being undetectable in G6PD deficient RBCs (G6PD-Mediterranean and G6PD-Genova). Indeed, Tyr-401 is located to a region of the G6PD molecule critical for the formation of the enzymatically active dimer. Amino acid replacements in this region are mostly associated with a chronic hemolysis phenotype. Using mutagenesis approach, we demonstrated that the phosphorylation status of Tyr401 modulates the interaction of G6PD with G6P and stabilizes G6PD in a catalytically more efficient conformation. RBCs from Fyn-/-mice are defective in G6PD activity, resulting in increased susceptibility to primaquine-induced intravascular hemolysis. This negatively affected the recycling of reduced Prx2 in response to oxidative stress, indicating that defective G6PD phosphorylation impairs defense against oxidation. In human RBCs, we confirm the involvement of the thioredoxin/Prx2 system in the increase vulnerability of G6PD deficient RBCs to oxidation. In conclusion, our data suggest that Fyn is an oxidative radical sensor, and that Fyn-mediated Tyr-401 phosphorylation, by increasing G6PD activity, plays an important role in the physiology of RBCs. Failure of G6PD activation by this mechanism may be a major limiting factor in the ability of G6PD deficient RBCs to withstand oxidative stress.
Keywords: G6PD; Oxidation; Primaquine; Red cells; Signaling.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Figures













References
-
- Cappellini M.D., Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. - PubMed
-
- Luzzatto L., Arese P. Favism and glucose-6-phosphate dehydrogenase deficiency. N. Engl. J. Med. 2018;378:1068–1069. - PubMed
-
- Naylor C.E., Rowland P., Basak A.K., Gover S., Mason P.J., Bautista J.M., Vulliamy T.J., Luzzatto L., Adams M.J. Glucose 6-phosphate dehydrogenase mutations causing enzyme deficiency in a model of the tertiary structure of the human enzyme. Blood. 1996;87:2974–2982. - PubMed
-
- Notaro R., Afolayan A., Luzzatto L. Human mutations in glucose 6-phosphate dehydrogenase reflect evolutionary history. Faseb. J. 2000;14:485–494. - PubMed
-
- Scott M.D., Wagner T.C., Chiu D.T. Decreased catalase activity is the underlying mechanism of oxidant susceptibility in glucose-6-phosphate dehydrogenase-deficient erythrocytes. Biochim. Biophys. Acta. 1993;1181:163–168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

