Low-protein/high-carbohydrate diet induces AMPK-dependent canonical and non-canonical thermogenesis in subcutaneous adipose tissue
- PMID: 32863211
- PMCID: PMC7358542
- DOI: 10.1016/j.redox.2020.101633
Low-protein/high-carbohydrate diet induces AMPK-dependent canonical and non-canonical thermogenesis in subcutaneous adipose tissue
Abstract
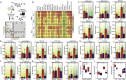
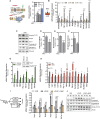
Low-protein/high-carbohydrate (LPHC) diet has been suggested to promote metabolic health and longevity in adult humans and animal models. However, the complex molecular underpinnings of how LPHC diet leads to metabolic benefits remain elusive. Through a multi-layered approach, here we observed that LPHC diet promotes an energy-dissipating response consisting in the parallel recruitment of canonical and non-canonical (muscular) thermogenic systems in subcutaneous white adipose tissue (sWAT). In particular, we measured Ucp1 induction in association with up-regulation of actomyosin components and several Serca (Serca1, Serca2a, Serca2b) ATPases. In beige adipocytes, we observed that AMPK activation is responsible for transducing the amino acid lowering in an enhanced fat catabolism, which sustains both Ucp1-and Serca-dependent energy dissipation. Limiting AMPK activation counteracts the expression of brown fat and muscular genes, including Ucp1 and Serca, as well as mitochondrial oxidative genes. We observed that mitochondrial reactive oxygen species are the upstream molecules controlling AMPK-mediated metabolic rewiring in amino acid-restricted beige adipocytes. Our findings delineate a novel metabolic phenotype of responses to amino acid shortage, which recapitulates some of the benefits of cool temperature in sWAT. In conclusion, this highlights LPHC diet as a valuable and practicable strategy to prevent metabolic diseases through the enhancement of mitochondrial oxidative metabolism and the recruitment of different energy dissipating routes in beige adipocytes.
Keywords: Metabolism; Mitochondria; Serca; Systems physiology; Ucp1.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors of this article just submitted to your journal, deny any financial or other relationships with drug manufacturers that could lead to a conflict of interest.
Figures




References
-
- Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H., Khandekar M., Virtanen K.A., Nuutila P., Schaart G., Huang K., Tu H., van Marken Lichtenbelt W.D., Hoeks J., Enerback S., Schrauwen P., Spiegelman B.M. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. - PMC - PubMed
-
- Petrovic N., Walden T.B., Shabalina I.G., Timmons J.A., Cannon B., Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 2010;285:7153–7164. - PMC - PubMed

