Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy
- PMID: 32863214
- PMCID: PMC7365984
- DOI: 10.1016/j.redox.2020.101635
Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy
Abstract
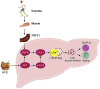
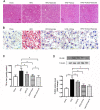
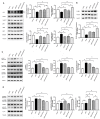
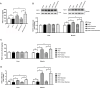
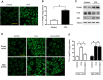
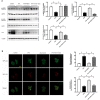
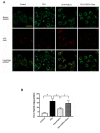
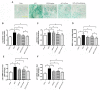
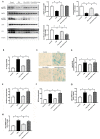
Exercise and dietary intervention are currently available strategies to treat nonalcoholic fatty liver disease (NAFLD), while the underlying mechanism remains controversial. Emerging evidence shows that lipophagy is involved in the inhibition of the lipid droplets accumulation. However, it is still unclear if exercise and dietary intervention improve NAFLD through regulating lipophagy, and how exercise of skeletal muscle can modulate lipid metabolism in liver. Moreover, NAFLD is associated with aging, and little is known about the effect of lipid accumulation on aging process. Here in vivo and in vitro models, we found that exercise and dietary intervention reduced lipid droplets formation, decreased hepatic triglyceride in the liver induced by high-fat diet. Exercise and dietary intervention enhanced the lipophagy by activating AMPK/ULK1 and inhibiting Akt/mTOR/ULK1 pathways respectively. Furthermore, exercise stimulated FGF21 production in the muscle, followed by secretion to the circulation to promote the lipophagy in the liver via an AMPK-dependent pathway. Importantly, for the first time, we demonstrated that lipid accumulation exacerbated liver aging, which was ameliorated by exercise and dietary intervention through inducing lipophagy. Our findings suggested a new mechanism of exercise and dietary intervention to improve NAFLD through promoting lipophagy. The study also provided evidence to support that muscle exercise is beneficial to other metabolic organs such as liver. The FGF21-mediated AMPK dependent lipophagy might be a potential drug target for NAFLD and aging caused by lipid metabolic dysfunction.
Keywords: Aging; Exercise; FGF21; Lipophagy; Nonalcoholic fatty liver disease (NAFLD).
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declared no conflict of interest.
Figures

References
-
- Anstee Q.M., Targher G., Day C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013;10(6):330–344. - PubMed
-
- Christian P., Sacco J., Adeli K. Autophagy: emerging roles in lipid homeostasis and metabolic control. Biochim. Biophys. Acta. 2013;1831(4):819–824. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

