Lessons from the post-genomic era: Globin diversity beyond oxygen binding and transport
- PMID: 32863222
- PMCID: PMC7475203
- DOI: 10.1016/j.redox.2020.101687
Lessons from the post-genomic era: Globin diversity beyond oxygen binding and transport
Abstract
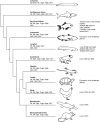
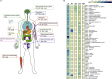
Vertebrate hemoglobin (Hb) and myoglobin (Mb) were among the first proteins whose structures and sequences were determined over 50 years ago. In the subsequent pregenomic period, numerous related proteins came to light in plants, invertebrates and bacteria, that shared the myoglobin fold, a signature sequence motif characteristic of a 3-on-3 α-helical sandwich. Concomitantly, eukaryote and bacterial globins with a truncated 2-on-2 α-helical fold were discovered. Genomic information over the last 20 years has dramatically expanded the list of known globins, demonstrating their existence in a limited number of archaeal genomes, a majority of bacterial genomes and an overwhelming majority of eukaryote genomes. In vertebrates, 6 additional globin types were identified, namely neuroglobin (Ngb), cytoglobin (Cygb), globin E (GbE), globin X (GbX), globin Y (GbY) and androglobin (Adgb). Furthermore, functions beyond the familiar oxygen transport and storage have been discovered within the vertebrate globin family, including NO metabolism, peroxidase activity, scavenging of free radicals, and signaling functions. The extension of the knowledge on globin functions suggests that the original roles of bacterial globins must have been enzymatic, involved in defense against NO toxicity, and perhaps also as sensors of O2, regulating taxis away or towards high O2 concentrations. In this review, we aimed to discuss the evolution and remarkable functional diversity of vertebrate globins with particular focus on the variety of non-canonical expression sites of mammalian globins and their according impressive variability of atypical functions.
Keywords: Cancer; Hemoglobin; Hypoxia; Myoglobin; Nitric oxide; Oxidative stress.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Gell D.A. Structure and function of haemoglobins. Blood Cells Mol. Dis. 2018;70:13–42. - PubMed
-
- de Sanctis D., Pesce A., Nardini M., Bolognesi M., Bocedi A., Ascenzi P. Structure-function relationships in the growing hexa-coordinate hemoglobin sub-family. IUBMB Life. 2004;56:643–651. - PubMed
-
- Kendrew J.C., Bodo G., Dintzis H.M., Parrish R.G., Wyckoff H., Phillips D.C. A three-dimensional model of the myoglobin molecule obtained by x-ray analysis. Nature. 1958;181:662–666. - PubMed
-
- Muirhead H., Perutz M.F. Structure of haemoglobin. A three-dimensional fourier synthesis of reduced human haemoglobin at 5-5 a resolution. Nature. 1963;199:633–638. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

