Nrf2/Wnt resilience orchestrates rejuvenation of glia-neuron dialogue in Parkinson's disease
- PMID: 32863224
- PMCID: PMC7395594
- DOI: 10.1016/j.redox.2020.101664
Nrf2/Wnt resilience orchestrates rejuvenation of glia-neuron dialogue in Parkinson's disease
Abstract
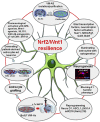
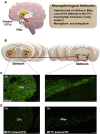
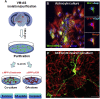
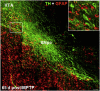
Oxidative stress and inflammation have long been recognized to contribute to Parkinson's disease (PD), a common movement disorder characterized by the selective loss of midbrain dopaminergic neurons (mDAn) of the substantia nigra pars compacta (SNpc). The causes and mechanisms still remain elusive, but a complex interplay between several genes and a number of interconnected environmental factors, are chiefly involved in mDAn demise, as they intersect the key cellular functions affected in PD, such as the inflammatory response, mitochondrial, lysosomal, proteosomal and autophagic functions. Nuclear factor erythroid 2 -like 2 (NFE2L2/Nrf2), the master regulator of cellular defense against oxidative stress and inflammation, and Wingless (Wnt)/β-catenin signaling cascade, a vital pathway for mDAn neurogenesis and neuroprotection, emerge as critical intertwinned actors in mDAn physiopathology, as a decline of an Nrf2/Wnt/β-catenin prosurvival axis with age underlying PD mutations and a variety of noxious environmental exposures drive PD neurodegeneration. Unexpectedly, astrocytes, the so-called "star-shaped" cells, harbouring an arsenal of "beneficial" and "harmful" molecules represent the turning point in the physiopathological and therapeutical scenario of PD. Fascinatingly, "astrocyte's fil rouge" brings back to Nrf2/Wnt resilience, as boosting the Nrf2/Wnt resilience program rejuvenates astrocytes, in turn (i) mitigating nigrostriatal degeneration of aged mice, (ii) reactivating neural stem progenitor cell proliferation and neuron differentiation in the brain and (iii) promoting a beneficial immunomodulation via bidirectional communication with mDAns. Then, through resilience of Nrf2/Wnt/β-catenin anti-ageing, prosurvival and proregenerative molecular programs, it seems possible to boost the inherent endogenous self-repair mechanisms. Here, the cellular and molecular aspects as well as the therapeutical options for rejuvenating glia-neuron dialogue will be discussed together with major glial-derived mechanisms and therapies that will be fundamental to the identification of novel diagnostic tools and treatments for neurodegenerative diseases (NDs), to fight ageing and nigrostriatal DAergic degeneration and promote functional recovery.
Keywords: Ageing; Astrocyte therapies; Gene-environment interactions; Glia-neuron crosstalk; Nrf2 signaling; Oxidative/inflammatory stress; Parkinson's disease; Wnt signaling.
Copyright © 2020 The Author. Published by Elsevier B.V. All rights reserved.
Figures



Similar articles
-
"Reframing" dopamine signaling at the intersection of glial networks in the aged Parkinsonian brain as innate Nrf2/Wnt driver: Therapeutical implications.Aging Cell. 2022 Apr;21(4):e13575. doi: 10.1111/acel.13575. Epub 2022 Mar 9. Aging Cell. 2022. PMID: 35262262 Free PMC article. Review.
-
Boosting Antioxidant Self-defenses by Grafting Astrocytes Rejuvenates the Aged Microenvironment and Mitigates Nigrostriatal Toxicity in Parkinsonian Brain via an Nrf2-Driven Wnt/β-Catenin Prosurvival Axis.Front Aging Neurosci. 2020 Mar 12;12:24. doi: 10.3389/fnagi.2020.00024. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32226376 Free PMC article.
-
Uncovering novel actors in astrocyte-neuron crosstalk in Parkinson's disease: the Wnt/β-catenin signaling cascade as the common final pathway for neuroprotection and self-repair.Eur J Neurosci. 2013 May;37(10):1550-63. doi: 10.1111/ejn.12166. Epub 2013 Mar 5. Eur J Neurosci. 2013. PMID: 23461676 Free PMC article. Review.
-
Reactive astrocytes are key players in nigrostriatal dopaminergic neurorepair in the MPTP mouse model of Parkinson's disease: focus on endogenous neurorestoration.Curr Aging Sci. 2013 Feb;6(1):45-55. doi: 10.2174/1874609811306010007. Curr Aging Sci. 2013. PMID: 23895521 Review.
-
Microglia Polarization, Gene-Environment Interactions and Wnt/β-Catenin Signaling: Emerging Roles of Glia-Neuron and Glia-Stem/Neuroprogenitor Crosstalk for Dopaminergic Neurorestoration in Aged Parkinsonian Brain.Front Aging Neurosci. 2018 Feb 12;10:12. doi: 10.3389/fnagi.2018.00012. eCollection 2018. Front Aging Neurosci. 2018. PMID: 29483868 Free PMC article. Review.
Cited by
-
Lactobacillus pentosus Alleviates Lipopolysaccharide-Induced Neuronal Pyroptosis via Promoting BIRC3-Mediated Inactivation of NLRC4.Evid Based Complement Alternat Med. 2022 Jun 23;2022:2124876. doi: 10.1155/2022/2124876. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35783533 Free PMC article.
-
High-Resolution Respirometry Reveals MPP+ Mitochondrial Toxicity Mechanism in a Cellular Model of Parkinson's Disease.Int J Mol Sci. 2020 Oct 22;21(21):7809. doi: 10.3390/ijms21217809. Int J Mol Sci. 2020. PMID: 33105548 Free PMC article.
-
Small Extracellular Vesicles Secreted by Nigrostriatal Astrocytes Rescue Cell Death and Preserve Mitochondrial Function in Parkinson's Disease.Adv Healthc Mater. 2022 Oct;11(20):e2201203. doi: 10.1002/adhm.202201203. Epub 2022 Aug 15. Adv Healthc Mater. 2022. PMID: 35856921 Free PMC article.
-
The impact of aging and oxidative stress in metabolic and nervous system disorders: programmed cell death and molecular signal transduction crosstalk.Front Immunol. 2023 Nov 8;14:1273570. doi: 10.3389/fimmu.2023.1273570. eCollection 2023. Front Immunol. 2023. PMID: 38022638 Free PMC article. Review.
-
Sinomenine Protects against Early Brain Injury by Inhibiting Microglial Inflammatory Response via Nrf2-Dependent Pathway after Subarachnoid Hemorrhage.Brain Sci. 2023 Apr 25;13(5):716. doi: 10.3390/brainsci13050716. Brain Sci. 2023. PMID: 37239188 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

