Asbestos conceives Fe(II)-dependent mutagenic stromal milieu through ceaseless macrophage ferroptosis and β-catenin induction in mesothelium
- PMID: 32863225
- PMCID: PMC7330611
- DOI: 10.1016/j.redox.2020.101616
Asbestos conceives Fe(II)-dependent mutagenic stromal milieu through ceaseless macrophage ferroptosis and β-catenin induction in mesothelium
Abstract
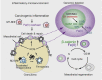
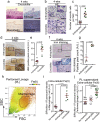
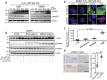
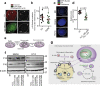
Asbestos is still a social burden worldwide as a carcinogen causing malignant mesothelioma. Whereas recent studies suggest that local iron reduction is a preventive strategy against carcinogenesis, little is known regarding the cellular and molecular mechanisms surrounding excess iron. Here by differentially using high-risk and low-risk asbestos fibers (crocidolite and anthophyllite, respectively), we identified asbestos-induced mutagenic milieu for mesothelial cells. Rat and cell experiments revealed that phagocytosis of asbestos by macrophages results in their distinctive necrotic death; initially lysosome-depenent cell death and later ferroptosis, which increase intra- and extra-cellular catalytic Fe(II). DNA damage in mesothelial cells, as assessed by 8-hydroxy-2'-deoxyguanosine and γ-H2AX, increased after crocidolite exposure during regeneration accompanied by β-catenin activation. Conversely, β-catenin overexpression in mesothelial cells induced higher intracellular catalytic Fe(II) with increased G2/M cell-cycle fraction, when p16INK4A genomic loci localized more peripherally in the nucleus. Mesothelial cells after challenge of H2O2 under β-catenin overexpression presented low p16INK4A expression with a high incidence of deletion in p16INK4A locus. Thus, crocidolite generated catalytic Fe(II)-rich mutagenic environment for mesothelial cells by necrotizing macrophages with lysosomal cell death and ferroptosis. These results suggest novel molecular strategies to prevent mesothelial carcinogenesis after asbestos exposure.
Keywords: Asbestos; Ferroptosis; Iron; Lysosomal cell death; p16(CDKN2A).
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest to present.
Figures




Similar articles
-
Ferroptosis-dependent extracellular vesicles from macrophage contribute to asbestos-induced mesothelial carcinogenesis through loading ferritin.Redox Biol. 2021 Nov;47:102174. doi: 10.1016/j.redox.2021.102174. Epub 2021 Oct 21. Redox Biol. 2021. PMID: 34700146 Free PMC article.
-
BRCA1 haploinsufficiency impairs iron metabolism to promote chrysotile-induced mesothelioma via ferroptosis resistance.Cancer Sci. 2023 Apr;114(4):1423-1436. doi: 10.1111/cas.15705. Epub 2023 Feb 2. Cancer Sci. 2023. PMID: 36541514 Free PMC article.
-
Iron addiction with ferroptosis-resistance in asbestos-induced mesothelial carcinogenesis: Toward the era of mesothelioma prevention.Free Radic Biol Med. 2019 Mar;133:206-215. doi: 10.1016/j.freeradbiomed.2018.10.401. Epub 2018 Oct 10. Free Radic Biol Med. 2019. PMID: 30312759 Review.
-
Frequent homozygous deletion of Cdkn2a/2b in tremolite-induced malignant mesothelioma in rats.Cancer Sci. 2020 Apr;111(4):1180-1192. doi: 10.1111/cas.14358. Epub 2020 Mar 21. Cancer Sci. 2020. PMID: 32080953 Free PMC article.
-
Decoding the molecular enigma behind asbestos and fibrous nanomaterial-induced carcinogenesis.J Occup Health. 2025 Jan 7;67(1):uiae064. doi: 10.1093/joccuh/uiae064. J Occup Health. 2025. PMID: 39871092 Free PMC article. Review.
Cited by
-
Interaction between macrophages and ferroptosis: Metabolism, function, and diseases.Chin Med J (Engl). 2025 Mar 5;138(5):509-522. doi: 10.1097/CM9.0000000000003189. Epub 2024 Sep 6. Chin Med J (Engl). 2025. PMID: 39245648 Free PMC article. Review.
-
Ferroptosis in tumor immunity and therapy.J Cell Mol Med. 2022 Nov;26(22):5565-5579. doi: 10.1111/jcmm.17529. Epub 2022 Nov 1. J Cell Mol Med. 2022. PMID: 36317423 Free PMC article. Review.
-
Double-edged Sword Role of Iron-loaded Ferritin in Extracellular Vesicles.J Cancer Prev. 2021 Dec 30;26(4):244-249. doi: 10.15430/JCP.2021.26.4.244. J Cancer Prev. 2021. PMID: 35047450 Free PMC article. Review.
-
Susceptibility of Brca1(L63X/+) rat to ovarian reserve dissipation by chemotherapeutic agents to breast cancer.Cancer Sci. 2025 Apr;116(4):1139-1152. doi: 10.1111/cas.16412. Epub 2025 Feb 3. Cancer Sci. 2025. PMID: 39901592 Free PMC article.
-
New Insights into Aspirin's Anticancer Activity: The Predominant Role of Its Iron-Chelating Antioxidant Metabolites.Antioxidants (Basel). 2024 Dec 29;14(1):29. doi: 10.3390/antiox14010029. Antioxidants (Basel). 2024. PMID: 39857363 Free PMC article. Review.
References
-
- Gualtieri A.F., Andreozzi G.B., Tomatis M., Turci F. Iron from a geochemical viewpoint. Understanding toxicity/pathogenicity mechanisms in iron-bearing minerals with a special attention to mineral fibers. Free Radic. Biol. Med. 2019;133:21–37. - PubMed
-
- Toyokuni S. Iron addiction with ferroptosis-resistance in asbestos-induced mesothelial carcinogenesis: toward the era of mesothelioma prevention. Free Radic. Biol. Med. 2019;133:206–215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

