Metabolic reprogramming sustains cancer cell survival following extracellular matrix detachment
- PMID: 32863227
- PMCID: PMC7371916
- DOI: 10.1016/j.redox.2020.101643
Metabolic reprogramming sustains cancer cell survival following extracellular matrix detachment
Abstract
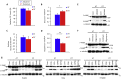
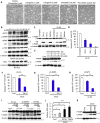
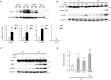
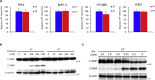
Epithelial cells require attachment to a support, such as the extracellular matrix, for survival. During cancer progression and metastasis, cancerous epithelial cells must overcome their dependence on adhesion signals. Dependence on glucose metabolism is a hallmark of cancer cells, but the nutrient requirements of cancer cells under anchorage-deficient conditions remain uncharacterized. Here, we report that cancer cells prioritize glutamine-derived tricarboxylic acid cycle energy metabolism over glycolysis to sustain anchorage-independent survival. Moreover, glutamine-dependent metabolic reprogramming is required not only to maintain ATP levels but also to suppress excessive oxidative stress through interaction with cystine. Mechanistically, AMPK, a central regulator of cellular responses to metabolic stress, participates in the induction of the expression of ASCT2, a glutamine transporter, and enhances glutamine consumption. Most interestingly, AMPK activation induces Nrf2 and its target proteins, allowing cancer cells to maintain energy homeostasis and redox status through glutaminolysis. Treatment with an integrin inhibitor was used to mimic the alterations in cell morphology and metabolic reprogramming caused by detachment. Under these conditions, cells were vulnerable to glutamine starvation or glutamine metabolism inhibitors. The observed preference for glutamine over glucose was more pronounced in aggressive cancer cell lines, and treatment with the glutaminase inhibitor, CB839, and cystine transporter inhibitor, sulfasalazine, caused strong cytotoxicity. Our data clearly show that anchorage-independent survival of cancer cells is supported mainly by glutaminolysis via the AMPK-Nrf2 signal axis. The discovery of new vulnerabilities along this route could help slow or prevent cancer progression.
Keywords: Anoikis; Extracellular matrix detachment; Glutaminolysis; Metabolic reprogramming; Metastasis.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

