Angiogenic and Antiangiogenic mechanisms of high density lipoprotein from healthy subjects and coronary artery diseases patients
- PMID: 32863238
- PMCID: PMC7364160
- DOI: 10.1016/j.redox.2020.101642
Angiogenic and Antiangiogenic mechanisms of high density lipoprotein from healthy subjects and coronary artery diseases patients
Abstract
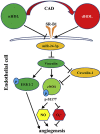
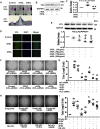
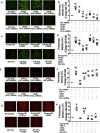
Normal high-density lipoprotein (nHDL) in normal, healthy subjects is able to promote angiogenesis, but the mechanism remains incompletely understood. HDL from patients with coronary artery disease may undergo a variety of oxidative modifications, rendering it dysfunctional; whether the angiogenic effect is mitigated by such dysfunctional HDL (dHDL) is unknown. We hypothesized that dHDL compromises angiogenesis. The angiogenic effects of nHDL and dHDL were assessed using endothelial cell culture, endothelial sprouts from cardiac tissue from C57BL/6 mice, zebrafish model for vascular growth and a model of impaired vascular growth in hypercholesterolemic low-density lipoprotein receptor null(LDLr-/-)mice. MiRNA microarray and proteomic analyses were used to determine the mechanisms. Lipid hydroperoxides were greater in dHDL than in nHDL. While nHDL stimulated angiogenesis, dHDL attenuated these responses. Protein and miRNA profiles in endothelial cells differed between nHDL and dHDL treatments. Moreover, nHDL suppressed miR-24-3p expression to increase vinculin expression resulting in nitric oxide (NO) production, whereas dHDL delivered miR-24-3p to inhibit vinculin expression leading to superoxide anion (O2•-) generation via scavenger receptor class B type 1. Vinculin was required for endothelial nitric oxide synthase (eNOS) expression and activation and modulated the PI3K/AKT/eNOS and ERK1/2 signaling pathways to regulate nHDL- and VEGF-induced angiogenesis. Vinculin overexpression or miR-24-3p inhibition reversed dHDL-impaired angiogenesis. The expressions of vinculin and eNOS and angiogenesis were decreased, but the expression of miR-24-3p and lipid hydroperoxides in HDL were increased in the ischemic lower limbs of hypercholesterolemic LDLr-/- mice. Overexpression of vinculin or miR-24-3p antagomir restored the impaired-angiogenesis in ischemic hypercholesterolemic LDLr-/- mice. Collectively, nHDL stimulated vinculin and eNOS expression to increase NO production by suppressing miR-24-3p to induce angiogenesis, whereas dHDL inhibited vinculin and eNOS expression to enhance O2•- generation by delivering miR-24-3p to impair angiogenesis, and that vinculin and miR-24-3p may be therapeutic targets for dHDL-impaired angiogenesis.
Keywords: Angiogenesis; Coronary artery disease; Endothelial nitric oxide synthase; High-density lipoprotein; Vinculin; miRNA.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures






References
-
- Ansell B.J., Navab M., Hama S. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108:2751–2756. - PubMed
-
- Miura S., Fujino M., Matsuo Y., Kawamura A., Tanigawa H., Nishikawa H., Saku K. High density lipoprotein-induced angiogenesis requires the activation of ras/map kinase in human coronary artery endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2003;23:802–808. - PubMed
-
- Seetharam D., Mineo C., Gormley A.K. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-b type i. Circ. Res. 2006;98:63–72. - PubMed
-
- Jin F., Hagemann N., Sun L., Wu J., Doeppner T.R., Dai Y., Hermann D.M. High-density lipoprotein (hdl) promotes angiogenesis via s1p3-dependent vegfr2 activation. Angiogenesis. 2018;21:381–394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

