Reactivity of terminal imido complexes of group 4-6 metals: stoichiometric and catalytic reactions involving cycloaddition with unsaturated organic molecules
- PMID: 32863399
- PMCID: PMC7453927
- DOI: 10.1016/j.ccr.2019.213118
Reactivity of terminal imido complexes of group 4-6 metals: stoichiometric and catalytic reactions involving cycloaddition with unsaturated organic molecules
Abstract
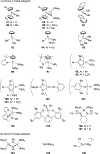
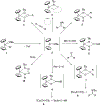
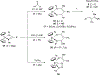
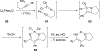
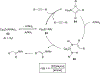
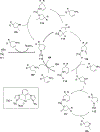
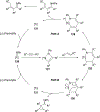
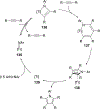
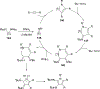
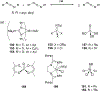
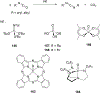
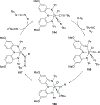
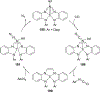
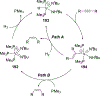
Imido complexes of early transition metals are key intermediates in the synthesis of many nitrogen-containing organic compounds. The metal-nitrogen double bond of the imido moiety undergoes [2+2] cycloaddition reactions with various unsaturated organic molecules to form new nitrogen-carbon and nitrogen-heteroatom bonds. This review article focuses on reactivity of the terminal imido complexes of Group 4-6 metals, summarizing their stoichiometric reactions and catalytic applications for a variety of reactions including alkyne hydroamination, alkyne carboamination, pyrrole formation, imine metathesis, and condensation reactions of carbonyl compounds with isocyanates.
Keywords: Cycloaddition; Early transition metals; Group 4—6 metals; Nitrogen-containing organic compound; Terminal imido complex.
Figures


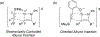
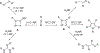




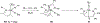
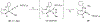

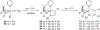




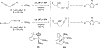

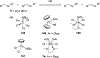


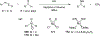

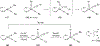
Similar articles
-
Enabling Nucleophilic Reactivity in High-Spin Fe(II) Imido Complexes: From Elementary Steps to Cooperative Catalysis.Acc Chem Res. 2023 Dec 5;56(23):3392-3403. doi: 10.1021/acs.accounts.3c00511. Epub 2023 Nov 13. Acc Chem Res. 2023. PMID: 37955993
-
Scandium Terminal Imido Chemistry.Acc Chem Res. 2018 Feb 20;51(2):557-566. doi: 10.1021/acs.accounts.7b00605. Epub 2018 Jan 30. Acc Chem Res. 2018. PMID: 29381048
-
Selective transformations of organic compounds by imidozirconocene complexes.Chem Rec. 2002;2(6):431-45. doi: 10.1002/tcr.10039. Chem Rec. 2002. PMID: 12469354
-
Transition metal-carboryne complexes: synthesis, bonding, and reactivity.Acc Chem Res. 2011 Apr 19;44(4):299-309. doi: 10.1021/ar100156f. Epub 2011 Mar 11. Acc Chem Res. 2011. PMID: 21395260 Review.
-
Manganese Alkyl Carbonyl Complexes: From Iconic Stoichiometric Textbook Reactions to Catalytic Applications.Acc Chem Res. 2022 Sep 20;55(18):2740-2751. doi: 10.1021/acs.accounts.2c00470. Epub 2022 Sep 8. Acc Chem Res. 2022. PMID: 36074912 Free PMC article. Review.
Cited by
-
Selective propargylic C(sp3)-H activation of methyl-substituted alkynes versus [2 + 2] cycloaddition at a titanium imido template.Chem Sci. 2021 Sep 28;12(41):13711-13718. doi: 10.1039/d1sc04334j. eCollection 2021 Oct 27. Chem Sci. 2021. PMID: 34760155 Free PMC article.
-
A mononuclear, terminal titanium(III) imido.Chem Commun (Camb). 2023 Aug 17;59(67):10101-10104. doi: 10.1039/d3cc01758c. Chem Commun (Camb). 2023. PMID: 37417771 Free PMC article.
-
N═N Bond Cleavage by Tantalum Hydride Complexes: Mechanistic Insights and Reactivity.Inorg Chem. 2022 Jan 10;61(1):474-485. doi: 10.1021/acs.inorgchem.1c03152. Epub 2021 Dec 10. Inorg Chem. 2022. PMID: 34890181 Free PMC article.
-
1,2-Addition and cycloaddition reactions of niobium bis(imido) and oxo imido complexes.Chem Sci. 2020 Oct 9;11(42):11613-11632. doi: 10.1039/d0sc03489d. Chem Sci. 2020. PMID: 34094408 Free PMC article.
-
Bonding and Reactivity of d0 Transition Metal Imido Complexes Encoded in Their 15N NMR Signatures.J Am Chem Soc. 2024 Apr 10;146(14):9860-9870. doi: 10.1021/jacs.3c14723. Epub 2024 Mar 27. J Am Chem Soc. 2024. PMID: 38534051 Free PMC article.
References
-
- Nugent WA, Haymore BL, Transition metal complexes containing organoimido (nr) and related ligands, Coord. Chem. Rev 31 (1980) 123–175. 10.1016/S0010-8545(00)80443-9; - DOI
- Wigley DE, Prog. Inorg. Chem 42 (1994) 239–482;
- Eikey RA, Abu-Omar MM, Nitrido and imido transition metal complexes of Group 6–8, Coord. Chem. Rev 243 (2003) 83–124. 10.1016/S0010-8545(03)00048-1. - DOI
- Schädle D, Anwander R, Rare-earth metal and actinide organoimide chemistry, Chem. Soc. Rev in press. - PubMed
-
- Schrock RR, Recent advances in high oxidation state Mo and W imido alkylidene chemistry, Chem. Rev 109 (2009) 3211–3226. 10.1021/cr800502p; - DOI - PMC - PubMed
- Schrock RR Recent advances in olefin metathesis by molybdenum and tungsten imido alkylidene complexes, J. Mol. Catal. A: Chem 213 (2004) 21–30. 10.1016/j.molcata.2003.10.060; - DOI - PubMed
- Schrock RR, Hoveyda AH, Molybdenum and tungsten imido alkylidene complexes as efficient olefin-metathesis catalysts, Angew. Chem., Int. Ed 42 (2003) 4592–4633. 10.1002/anie.200300576. - DOI - PubMed
-
- Bolton PD, Mountford P, Transition metal imido compounds as Ziegler-Natta olefin polymerization catalysts, Adv. Synth. Catal 347 (2005) 355–366. 10.1002/adsc.200404267. - DOI
-
- Webb JR, Burgess SA, Cundari TR, Gunnoe TB, Activation of carbon—hydrogen bonds ad dihydrogen by 1,2-CH-addition across metal—hetero atom bonds, Dalton Trans. 42 (2013) 16646–16665. 10.1039/C3DT52164H; - DOI - PubMed
- Wolczanski PT, Activation of carbon—hydrogen bonds via 1,2-RH-addition/-elimination to early transition metal imides, Organometallics 37 (2018) 505–516. 10.1021/acs.organomet.7b00753. - DOI
