MATRIX-BASED CONTROLLED RELEASE DELIVERY OF ACYCLOVIR FROM POLY-(ETHYLENE CO-VINYL ACETATE) RINGS
- PMID: 32863890
- PMCID: PMC7451249
- DOI: 10.1016/j.jddst.2019.101391
MATRIX-BASED CONTROLLED RELEASE DELIVERY OF ACYCLOVIR FROM POLY-(ETHYLENE CO-VINYL ACETATE) RINGS
Abstract
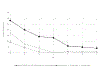
Up to 85% of the US adult population carries herpes simplex virus type-1 (HSV-1), with a smaller percentage (22%) infected with HSV-2. Herpesviruses can survive in lytic phase, when the viruses are actively replicating, or in latency, when the virus is functionally dormant in ganglia. Among drugs to treat these infections is acyclovir (ACV). ACV exhibits poor oral bioavailability and a short in vivo half-life; only about 10-15% of ingested drug enters the bloodstream and its half-life is about 3 hours. With those disadvantages and the possibility of poor patient compliance, viral replication may not always be suppressed. To abrogate these shortcomings we propose local distribution via sustained drug release. We present a matrix-based antiherpetic ring, composed of poly(ethylene co-vinyl acetate), that releases ACV directly to the vaginal epithelium. A 30-day in vitro drug release trial showed that approximately 135 +/- 20 μg/day of ACV was consistently released. Rings were nontoxic in cell culture and suppressed primary HSV-1 and HSV-2 replication. We expect these data form the basis for novel interventions in human health, where new prophylactics and therapeutics against genital herpes are truly needed.
Keywords: Herpes; acyclovir; controlled release; genital herpes; poly(ethylene-co-vinyl acetate); vaginal ring.
Figures



Similar articles
-
3D-Printed EVA Devices for Antiviral Delivery and Herpes Virus Control in Genital Infection.Viruses. 2022 Nov 11;14(11):2501. doi: 10.3390/v14112501. Viruses. 2022. PMID: 36423110 Free PMC article.
-
The effect of gramicidin, a topical contraceptive and antimicrobial agent with anti-HIV activity, against herpes simplex viruses type 1 and 2 in vitro.Arch Virol. 1997;142(11):2225-35. doi: 10.1007/s007050050237. Arch Virol. 1997. PMID: 9672588
-
Simultaneous delivery of tenofovir and acyclovir via an intravaginal ring.Antimicrob Agents Chemother. 2012 Feb;56(2):875-82. doi: 10.1128/AAC.05662-11. Epub 2011 Nov 28. Antimicrob Agents Chemother. 2012. PMID: 22123689 Free PMC article.
-
Acyclovir: a new use for an old drug.Curr Opin Infect Dis. 2009 Dec;22(6):583-7. doi: 10.1097/QCO.0b013e32833229b8. Curr Opin Infect Dis. 2009. PMID: 19726982 Review.
-
Current status and prospects for oral acyclovir treatment of first episode and recurrent genital herpes simplex virus.J Antimicrob Chemother. 1983 Sep;12 Suppl B:61-5. doi: 10.1093/jac/12.suppl_b.61. J Antimicrob Chemother. 1983. PMID: 6355052 Review.
Cited by
-
Instantaneous Inactivation of Herpes Simplex Virus by Silicon Nitride Bioceramics.Int J Mol Sci. 2023 Aug 10;24(16):12657. doi: 10.3390/ijms241612657. Int J Mol Sci. 2023. PMID: 37628838 Free PMC article.
-
Next-generation 3D printed multipurpose prevention intravaginal ring for prevention of HIV, HSV-2, and unintended pregnancy.J Control Release. 2024 Dec;376:1209-1224. doi: 10.1016/j.jconrel.2024.10.059. Epub 2024 Nov 12. J Control Release. 2024. PMID: 39500407 Free PMC article.
-
3D-Printed EVA Devices for Antiviral Delivery and Herpes Virus Control in Genital Infection.Viruses. 2022 Nov 11;14(11):2501. doi: 10.3390/v14112501. Viruses. 2022. PMID: 36423110 Free PMC article.
-
The Vaginal Microbiota, Bacterial Biofilms and Polymeric Drug-Releasing Vaginal Rings.Pharmaceutics. 2021 May 19;13(5):751. doi: 10.3390/pharmaceutics13050751. Pharmaceutics. 2021. PMID: 34069590 Free PMC article. Review.
-
Pseudoboehmite as a drug delivery system for acyclovir.Sci Rep. 2021 Jul 29;11(1):15448. doi: 10.1038/s41598-021-94325-y. Sci Rep. 2021. PMID: 34326377 Free PMC article.
References
-
- Acosta EP, & Flexner C (2011). Antiviral Agents (Nonretroviral). In Goodman & Gilman ‘s The Pharmacological Basis of Therapeutics.
Grants and funding
LinkOut - more resources
Full Text Sources

