DNA methylation downregulated ZDHHC1 suppresses tumor growth by altering cellular metabolism and inducing oxidative/ER stress-mediated apoptosis and pyroptosis
- PMID: 32863941
- PMCID: PMC7449911
- DOI: 10.7150/thno.45631
DNA methylation downregulated ZDHHC1 suppresses tumor growth by altering cellular metabolism and inducing oxidative/ER stress-mediated apoptosis and pyroptosis
Abstract
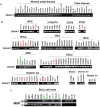
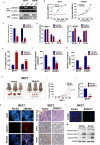
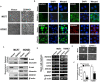
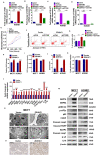
Cancer progression is an intricate biological process profiled by not only unscheduled proliferation, but also altered metabolism mechanisms. In this article, we introduced a novel tumor suppressor gene (TSG), Zinc Finger DHHC-Type Containing 1 (ZDHHC1, also known as ZNF377), frequently silenced due to epigenetic modification among various cancers, which exerts significant anti-tumor effects through metabolic regulation. Methods: Quantitative reversed-transcription PCR (qRT-PCR), reverse transcription PCR (RT-PCR) and Western blot were employed to demonstrate transcriptional and protein levels of targeted regulators. Methylation of ZDHHC1 promoter was detected by bisulfite genomic sequencing (BGS) and methylation specific PCR (MSP). Proteomics were analyzed by isobaric tags for relative and absolute quantitation (iTRAQ) and gas chromatography-mass spectrometry (GC-MS) were utilized for metabolomics analysis. Cellular functions were examined via corresponding approaches. Nude mice were used for xenograft tumor models. Indirect immunofluorescence staining was utilized to obtain precise location and expression of target proteins. Oxidative and ER stress indicators were detected using specific kits. Results: We found that ZDHHC1 expression was frequently silenced in multiple tumor cells and specimens due to methylation. Restoration of ZDHHC1 expression can curb cancer cell progression via stimulating apoptosis and cell cycle arrest, repressing metastasis, and reversing EMT transition and cell stemness. ZDHHC1's salient anti-tumor abilities were recognized in vivo as well. Metabolomic and proteomic analyses predicted inhibitory role of ZDHHC1 in glucose metabolism pathways in a CYGB-dependent manner, and in pentose phosphate pathway (PPP), which was validated by examining altered key factors. Moreover, we unraveled that ZDHHC1 dedicates to the increment of oxidative stress and endoplasmic reticulum (ER) stress to promote pyroptosis for anticancer purposes. Conclusion: Our study for the first time indicates ZDHHC1 is a potential tumor-suppressor frequently silenced due to promoter methylation, capable of negatively regulating metabolisms of tumor cells while stimulating oxidative stress and ER stress to expedite cell death through induction of pyroptosis and apoptosis, which can be exploited for development of new cancer prevention and therapies.
Keywords: CYGB; ER stress; Oxidative stress; ZDHHC1; zinc finger protein.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

