Low-intensity Pulsed Ultrasound regulates alveolar bone homeostasis in experimental Periodontitis by diminishing Oxidative Stress
- PMID: 32863960
- PMCID: PMC7449900
- DOI: 10.7150/thno.42508
Low-intensity Pulsed Ultrasound regulates alveolar bone homeostasis in experimental Periodontitis by diminishing Oxidative Stress
Erratum in
-
Erratum: Low-intensity Pulsed Ultrasound regulates alveolar bone homeostasis in experimental Periodontitis by diminishing Oxidative Stress: Erratum.Theranostics. 2022 Jan 1;12(3):1337-1340. doi: 10.7150/thno.69529. eCollection 2022. Theranostics. 2022. PMID: 35154492 Free PMC article.
Abstract
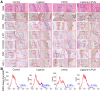
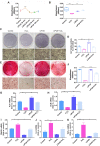
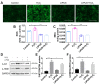
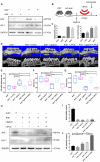
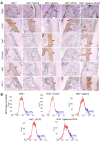
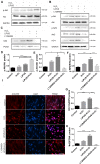
Periodontitis is a widespread oral disease that results in the loss of alveolar bone. Low-intensity pulsed ultrasound (LIPUS), which is a new therapeutic option, promotes alveolar bone regeneration in periodontal bone injury models. This study investigated the protective effect of LIPUS on oxidative stress in periodontitis and the mechanism underlying this process. Methods: An experimental periodontitis model was induced by administering a ligature. Immunohistochemistry was performed to detect the expression levels of oxidative stress, osteogenic, and osteoclastogenic markers in vivo. Cell viability and osteogenic differentiation were analyzed using the Cell Counting Kit-8, alkaline phosphatase, and Alizarin Red staining assays. A reactive oxygen species assay kit, lipid peroxidation MDA assay kit, and western blotting were used to determine oxidative stress status in vitro. To verify the role of nuclear factor erythroid 2-related factor 2 (Nrf2), an oxidative regulator, during LIPUS treatment, the siRNA technique and Nrf2-/- mice were used. The PI3K/Akt inhibitor LY294002 was utilized to identify the effects of the PI3K-Akt/Nrf2 signaling pathway. Results: Alveolar bone resorption, which was experimentally induced by periodontitis in vivo, was alleviated by LIPUS via activation of Nrf2. Oxidative stress, induced via H2O2 treatment in vitro, inhibited cell viability and suppressed osteogenic differentiation. These effects were also alleviated by LIPUS treatment via Nrf2 activation. Nrf2 silencing blocked the antioxidant effect of LIPUS by diminishing heme oxygenase-1 expression. Nrf2-/- mice were susceptible to ligature-induced periodontitis, and the protective effect of LIPUS on alveolar bone dysfunction was weaker in these mice. Activation of Nrf2 by LIPUS was accompanied by activation of the PI3K/Akt pathway. The oxidative defense function of LIPUS was inhibited by exposure to LY294002 in vitro. Conclusions: These results demonstrated that LIPUS regulates alveolar bone homeostasis in periodontitis by attenuating oxidative stress via the regulation of PI3K-Akt/Nrf2 signaling. Thus, Nrf2 plays a pivotal role in the protective effect exerted by LIPUS against ligature-induced experimental periodontitis.
Keywords: alveolar bone; oxidative stress; periodontitis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Page RC, Kornman KS. The pathogenesis of human periodontitis: an introduction. Periodontol 2000. 1997;14:9–11. - PubMed
-
- Saita M, Kaneko J, Sato T, Takahashi SS, Wada-Takahashi S, Kawamata R. et al. Novel antioxidative nanotherapeutics in a rat periodontitis model: Reactive oxygen species scavenging by redox injectable gel suppresses alveolar bone resorption. Biomaterials. 2016;76:292–301. - PubMed
-
- Ekuni D, Tomofuji T, Tamaki N, Sanbe T, Azuma T, Yamanaka R. et al. Mechanical stimulation of gingiva reduces plasma 8-OHdG level in rat periodontitis. Arch Oral Biol. 2008;53:324–9. - PubMed
-
- Gandhi KK, Pavaskar R, Cappetta EG, Drew HJ. Effectiveness of Adjunctive Use of Low-Level Laser Therapy and Photodynamic Therapy After Scaling and Root Planing in Patients with Chronic Periodontitis. Int J Periodontics Restorative Dent. 2019;39:837–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

