Advanced 4D Bioprinting Technologies for Brain Tissue Modeling and Study
- PMID: 32864037
- PMCID: PMC7451241
- DOI: 10.1080/19475411.2019.1631899
Advanced 4D Bioprinting Technologies for Brain Tissue Modeling and Study
Abstract
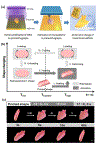
Although the process by which the cortical tissues of the brain fold has been the subject of considerable study and debate over the past few decades, a single mechanistic description of the phenomenon has yet to be fully accepted. Rather, two competing explanations of cortical folding have arisen in recent years; known as the axonal tension and the differential tangential expansion models. In the present review, these two models are introduced by analyzing the computational, theoretical, materials-based, and cell studies which have yielded them. Then Four-dimensional bioprinting is presented as a powerful technology which can not only be used to test both models of cortical folding de novo, but can also be used to explore the reciprocal effects that folding associated mechanical stresses may have on neural development. Therein, the fabrication of "smart" tissue models which can accurately simulate the in vivo folding process and recapitulate physiologically relevant stresses are introduced. We also provide a general description of both cortical neurobiology as well as the cellular basis of cortical folding. Our discussion also entails an overview of both 3D and 4D bioprinting technologies, as well as a brief commentary on recent advancements in printed central nervous system tissue engineering.
Keywords: 4D Bioprinting; Brain; Cortical folding; Foliation; Gyrification; Organoids; Smart materials.
Conflict of interest statement
Disclosure Statement The authors declare no conflicts of interest.
Figures

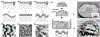




References
-
- Armstrong E, et al. (1995). “The Ontogeny of Human Gyrification.” Cerebral Cortex 5(1): 56–63. - PubMed
-
- Sekiguchi M, et al. (1991). “Abnormalities of foliation and neuronal position in the cerebellum of NZB/BINJ mouse.” Developmental Brain Research 64(1): 189–195. - PubMed
-
- Wallace Gregory L.; Robustelli Briana; Dankner Nathan; Kenworthy Lauren; Giedd Jay N.; Martin Alex (2013-June-01). “Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders”. Brain. 136 (6): 1956–1967. doi: 10.1093/brain/awt106. ISSN 0006–8950. PMC 3673467 Freely accessible. PMID 23715094. - DOI - PMC - PubMed
-
- Harris JM, et al. (2007). “Increased Prefrontal Gyrification in a Large High-Risk Cohort Characterizes Those Who Develop Schizophrenia and Reflects Abnormal Prefrontal Development.” Biological Psychiatry 62(7): 722–729. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
