Long-term Outcomes of Treat and Extend Regimen of Anti-vascular Endothelial Growth Factor in Neovascular Age-related Macular Degeneration
- PMID: 32864064
- PMCID: PMC7431723
- DOI: 10.18502/jovr.v15i3.7452
Long-term Outcomes of Treat and Extend Regimen of Anti-vascular Endothelial Growth Factor in Neovascular Age-related Macular Degeneration
Abstract
Purpose: This study describes the long-term visual and anatomic outcomes of anti-vascular endothelial growth factor (VEGF) treatment using a treat and extend dosing regimen.
Methods: This cross-sectional cohort study consisted of 224 treatment-naïve eyes with neovascular age-related macular degeneration (NV-AMD) from 202 patients that were treated with anti-VEGF agents bevacizumab, ranibizumab, and aflibercept using a treat and extend (TAE) regimen by four physician investigators in a large urban referral center from 2008 to 2015. Subjects were evaluated for visual acuity, injection frequency, and optical coherence tomography (OCT).
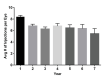
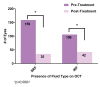
Results: Over a seven-year follow-up period (mean 3.4 years), an average 20.2 14.7 injections were administered with 8.4 injections in the first year and 5.5 injections by the seventh year of remaining eyes undergoing treatment. Visual acuity was 0.70 logMAR (20/100 Snellen) at the first visit and 0.67 logMAR (20/93 Snellen) at the final visit, with 74% of eyes maintaining or gaining more than 2 lines of vision. Long-term, 45.1% of eyes achieved 20/50 or better, while 27.1% were 20/200 or worse. Of the treated patients, 61.2% received monotherapy with no difference in visual acuity outcomes or number of injections between the agents used. OCT analysis showed decreased fluid from initial to final follow-up visit: 70.1-15.6% with sub-retinal fluid (SRF) and 47.3-18.8% with intra-retinal fluid (IRF) with no difference between the agents were used.
Conclusion: This study demonstrates that most patients (74%) improve or maintain visual acuity long-term using a TAE model with a significant portion (45.1%) achieving 20/50 or better visual acuity with sustained treatment.
Keywords: Intraocular Drugs; Visual Acuity; Age-related Macular Degeneration (AMD).
Copyright © 2020 Lee et al.
Conflict of interest statement
There are no conflicts of interest.
Figures


References
-
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. New Engl J Med 2006;355:1419–1431. - PubMed
-
- Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol 2009;148:43–58e1. - PubMed
-
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 2009;116:57–65e5. - PubMed
-
- Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012;119:2537–2548. - PubMed
-
- Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology 2014;121:193–201. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
