Safety of Intravitreal Injection of Stivant, a Biosimilar to Bevacizumab, in Rabbit Eyes
- PMID: 32864065
- PMCID: PMC7431730
- DOI: 10.18502/jovr.v15i3.7453
Safety of Intravitreal Injection of Stivant, a Biosimilar to Bevacizumab, in Rabbit Eyes
Abstract
Purpose: To evaluate the safety of intravitreal injection of Stivant, a biosimilar to bevacizumab, in rabbits using electrophysiological and histological analysis.
Methods: Both eyes of 41 New Zealand albino rabbits were injected with 0.1 mL (2.5 mg) of Stivant. The rabbits were scheduled to be sacrificed 1, 2, 7, 14, and 28 days after injection for histopathological evaluations. Clinical examinations and electroretinography (ERG) were performed at baseline and just before sacrificing the rabbits. Fourteen separate rabbits received a reference drug (Avastin) and were considered as the control group. Furthermore, three other rabbits received the same volume of saline (saline control group). Rabbits of both control groups were sacrificed four weeks after injection. ERG was performed 1, 2, 7, 14, and 28 days after injections.
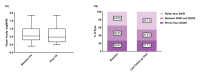
Results: No significant difference was observed in a- and b-wave amplitudes and latency after intravitreal Stivant injection between baseline and different time points. Moreover, there was no statistically significant difference in wave amplitudes and latency between the Stivant and control groups. The histology of rabbit eyes of the Stivant and control groups after intravitreal injections was not distinguishable.
Conclusion: The biosimilar Stivant, up to a dose of 2.5 mg, did not appear to be toxic to the retina in albino rabbits. These results suggest that this drug could be a safe and inexpensive alternative to intravitreal bevacizumab. The efficacy of these injections was not investigated in this study and needs to be evaluated in future studies.
Keywords: Intravitreal Injection; Safety; Stivant; Biosimilar.
Copyright © 2020 Lashay et al.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Shinoda K, Ishida S, Kawashima S, Wakabayashi T, Uchita M, Matsuzaki T, et al. Clinical factors related to the aqueous levels of vascular endothelial growth factor and hepatocyte growth factor in proliferative diabetic retinopathy. Curr Eye Res 2000;21:655–661. - PubMed
-
- Rezzola S, Nawaz MI, Cancarini A, Semeraro F, Presta M. Vascular Endothelial Growth Factor in the vitreous of proliferative diabetic retinopathy patients: chasing a hiding prey? Diabetes Care 2019;42:e105–e106. - PubMed
-
- Hera R, Keramidas M, Peoc'h M, Mouillon M, Romanet JP, Feige JJ. Expression of VEGF and angiopoietins in subfoveal membranes from patients with age-related macular degeneration. Am J Ophthalmol 2005;139:589–596. - PubMed
-
- Vuletic I, Zhou K, Li H, Bai H, Meng X, Zhu S, et al. Validation of bevacizumab therapy effect on colon cancer subtypes by using whole body imaging in mice. Mol Imaging Biol 2017;19:847–856. - PubMed
-
- Maberley DAL, Zhang R, Ding L, Flatt AH, Etminan M, Hewitt M. One-year effectiveness study of intravitreous bevacizumab in neovascular age-related macular degeneration: a population-based retrospective cohort study. Can J Ophthalmol 2018;53:627–631. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
