Antibacterial activity and mechanism of sanguinarine against Providencia rettgeri in vitro
- PMID: 32864203
- PMCID: PMC7427548
- DOI: 10.7717/peerj.9543
Antibacterial activity and mechanism of sanguinarine against Providencia rettgeri in vitro
Abstract
Background: Sanguinarine (SAG), a benzophenanthridine alkaloid, occurs in Papaveraceas, Berberidaceae and Ranunculaceae families. Studies have found that SAG has antioxidant, anti-inflammatory, and antiproliferative activities in several malignancies and that it exhibits robust antibacterial activities. However, information reported on the action of SAG against Providencia rettgeri is limited in the literature. Therefore, the present study aimed to evaluate the antimicrobial and antibiofilm activities of SAG against P. rettgeri in vitro.
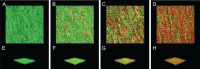
Methods: The agar dilution method was used to determine the minimum inhibitory concentration (MIC) of SAG against P. rettgeri. The intracellular ATP concentration, intracellular pH (pHin), and cell membrane integrity and potential were measured. Confocal laser scanning microscopy (CLSM), field emission scanning electron microscopy (FESEM), and crystal violet staining were used to measure the antibiofilm formation of SAG.
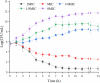
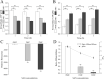
Results: The MIC of SAG against P. rettgeri was 7.8 μg/mL. SAG inhibited the growth of P. rettgeri and destroyed the integrity of P. rettgeri cell membrane, as reflected mainly through the decreases in the intracellular ATP concentration, pHin and cell membrane potential and significant changes in cellular morphology. The findings of CLSM, FESEM and crystal violet staining indicated that SAG exhibited strong inhibitory effects on the biofilm formation of P. rettgeri and led to the inactivity of biofilm-related P. rettgeri cells.
Keywords: Antibiofilm; Antimicrobial; Providencia rettgeri; Sanguinarine.
© 2020 Zhang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Armbruster CE, Smith SN, Yep A, Mobley HLT. Increased incidence of urolithiasis and bacteremia during Proteus mirabilis and Providencia stuartii coinfection due to synergistic induction of urease activity. Journal of Infectious Diseases. 2014;209(10):1524–1532. doi: 10.1093/infdis/jit663. - DOI - PMC - PubMed
-
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Dieases (ESCMID) EUCAST definitive document E.DEF 3.1, June 2000: determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2000;6(9):509–515. doi: 10.1046/j.1469-0691.2000.00142.x. - DOI - PubMed
-
- Giaouris E, Heir E, Hébraud M, Chorianopoulos N, Langsrud S, Møretrø T, Habimana O, Desvaux M, Renier S, Nychas G-J. Attachment and biofilm formation by foodborne bacteria in meat processing environments: causes, implications, role of bacterial interactions and control by alternative novel methods. Meat Science. 2014;97(3):298–309. doi: 10.1016/j.meatsci.2013.05.023. - DOI - PubMed
LinkOut - more resources
Full Text Sources

