Antibiotic eluting sinus stents
- PMID: 32864430
- PMCID: PMC7444760
- DOI: 10.1002/lio2.423
Antibiotic eluting sinus stents
Abstract
Objectives: Chronic rhinosinusitis (CRS) is a multifactorial disease affecting up to 16% of the United States population and disproportionately affecting the cystic fibrosis (CF) patient population. Despite treating the underlying infection, the use of systemic antibiotics has shown little efficacy in alleviation of symptom burden. This review seeks to discuss recent research on novel antibiotic eluting stent therapy in vitro and within animal models as well as the factors that contribute to its efficacy.
Data sources: PubMed literature review.
Review methods: A review of all published literature related to antibiotic eluting sinus stents was conducted to integrate and summarize this innovative approach to chronic sinus infections.
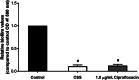
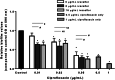
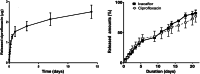
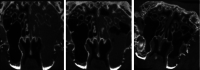
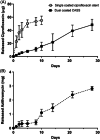
Results: Placement of the ciprofloxacin sinus stent (CSS) and ciprofloxacin-ivacaftor sinus stent (CISS) exhibited improvement in endoscopic and radiographic findings in rabbit CRS models. While the CSS showed an overall trend toward improvement in microscopic findings and a reduction in biofilm mass, there remained a significant quantity of planktonic bacteria due to antibiotic depletion from an initial burst release in the first 48 hours of stent placement. The CISS and ciprofloxacin-azithromycin sinus stents (CASSs) exhibited controlled antibiotic release over the study period leading to greatly reduced planktonic bacterial load and biofilm mass. In vitro studies indicate that CASS may be just as efficacious at reducing biofilm mass.
Conclusion: Antibiotic eluting sinus stents show significant promise as a novel therapeutic strategy for CRS. The CISS may have particular promise for the CF patient population by addressing both the infectious and genetic components of disease. Animal studies demonstrate significant promise for translation into human studies. Human clinical trials are warranted to determine the efficacy of antibiotic sinus stents in human patients.
Level of evidence: NA.
Keywords: antibiotic stent; azithromycin; biofilm; chronic rhinosinusitis; ciprofloxacin; cystic fibrosis; ivacaftor; sinus stent; sinusitis; stent.
© 2020 The Authors. Laryngoscope Investigative Otolaryngology published by Wiley Periodicals LLC. on behalf of The Triological Society.
Conflict of interest statement
B. A. W. is a consultant for Cook Medical, Smith and Nephew, and Baxter.
Figures

References
-
- Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(suppl 1):S22‐S209. - PubMed
-
- DeConde AS, Soler ZM. Chronic rhinosinusitis: epidemiology and burden of disease. Am J Rhinol Allergy. 2016;30(2):134‐139. - PubMed
-
- Blackwell DL, Collins JG, Coles R. Summary health statistics for U.S. adults: National Health Interview Survey, 1997. Vital Health Stat. 2002;10(205):1‐109. - PubMed
-
- Bhattacharyya N, Orlandi RR, Grebner J, Martinson M. Cost burden of chronic rhinosinusitis: a claims‐based study. Otolaryngol Head Neck Surg. 2011;144(3):440‐445. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
