Development and Clinical Application of Phosphorus-Containing Drugs
- PMID: 32864606
- PMCID: PMC7445155
- DOI: 10.1016/j.medidd.2020.100063
Development and Clinical Application of Phosphorus-Containing Drugs
Abstract
Phosphorus-containing drugs belong to an important class of therapeutic agents and are widely applied in daily clinical practices. Structurally, the phosphorus-containing drugs can be classified into phosphotriesters, phosphonates, phosphinates, phosphine oxides, phosphoric amides, bisphosphonates, phosphoric anhydrides, and others; functionally, they are often designed as prodrugs with improved selectivity and bioavailability, reduced side effects and toxicity, or biomolecule analogues with endogenous materials and antagonistic endoenzyme supplements. This review summarized the phosphorus-containing drugs currently on the market as well as a few promising molecules at clinical studies, with particular emphasis on their structural features, biological mechanism, and indications.
Keywords: Drug modification; Phosphite, phosphate; Phosphorus-containing drugs; Prodrugs.
© 2020 The Authors.
Conflict of interest statement
The authors declare that they do not have any conflicts of interest.
Figures


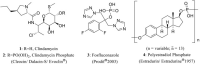
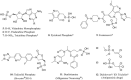

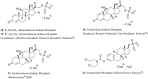
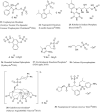
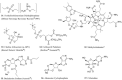



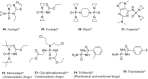


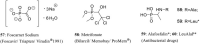



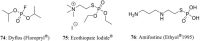


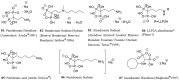

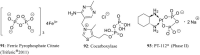


References
-
- Andrew W. Pharmaceutical Manufacturing Encyclopedia. 3rd ed.. William Andrew Publishing, Norwish NY: Elsevier; 2007; p. 56m-57m. ISBN-13: 9870815515265.
-
- Yao Q., Reng L., Ran M., He J., Xiang D. Review on the structures of phosphorus-containing drugs used in clinical practice. Hua xue Shi ji. 2019;41:139–146. doi: 10.13822/j.cnki.hxsj.2019006737. - DOI
-
- Karaman R. 1th ed. Nova Science Publisher, Inc; Nova Biomedical, New York: 2014. Prodrugs Design: A New Era (Pharmacology- Research, Safety Testing and Reaction) pp. 112–115. ISBN: 9781631177019.
Publication types
LinkOut - more resources
Full Text Sources
