Fusion-Independent Satellite Cell Communication to Muscle Fibers During Load-Induced Hypertrophy
- PMID: 32864621
- PMCID: PMC7448100
- DOI: 10.1093/function/zqaa009
Fusion-Independent Satellite Cell Communication to Muscle Fibers During Load-Induced Hypertrophy
Abstract
The "canonical" function of Pax7+ muscle stem cells (satellite cells) during hypertrophic growth of adult muscle fibers is myonuclear donation via fusion to support increased transcriptional output. In recent years, however, emerging evidence suggests that satellite cells play an important secretory role in promoting load-mediated growth. Utilizing genetically modified mouse models of delayed satellite cell fusion and in vivo extracellular vesicle (EV) tracking, we provide evidence for satellite cell communication to muscle fibers during hypertrophy. Myogenic progenitor cell-EV-mediated communication to myotubes in vitro influences extracellular matrix (ECM)-related gene expression, which is congruent with in vivo overload experiments involving satellite cell depletion, as well as in silico analyses. Satellite cell-derived EVs can transfer a Cre-induced, cytoplasmic-localized fluorescent reporter to muscle cells as well as microRNAs that regulate ECM genes such as matrix metalloproteinase 9 (Mmp9), which may facilitate growth. Delayed satellite cell fusion did not limit long-term load-induced muscle hypertrophy indicating that early fusion-independent communication from satellite cells to muscle fibers is an underappreciated aspect of satellite cell biology. We cannot exclude the possibility that satellite cell-mediated myonuclear accretion is necessary to maintain prolonged growth, specifically in the later phases of adaptation, but these data collectively highlight how EV delivery from satellite cells can directly contribute to mechanical load-induced muscle fiber hypertrophy, independent of cell fusion to the fiber.
Keywords: Mmp9; Nr4a1; Pax7-DTA; extracellular vesicles; miRNA; tdTomato.
© American Physiological Society 2020.
Figures
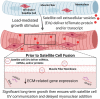


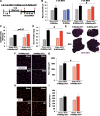
Comment in
-
New Insight into a Classic Stem Cell: the Satellite Cell may Communicate with the Muscle Fiber via Extracellular Vesicles-A Perspective on "Fusion-Independent Satellite Cell Communication to Muscle Fibers During Load-Induced Hypertrophy".Function (Oxf). 2020 Sep 3;1(2):zqaa015. doi: 10.1093/function/zqaa015. eCollection 2020. Function (Oxf). 2020. PMID: 35330641 Free PMC article. No abstract available.
Similar articles
-
Early satellite cell communication creates a permissive environment for long-term muscle growth.iScience. 2021 Mar 29;24(4):102372. doi: 10.1016/j.isci.2021.102372. eCollection 2021 Apr 23. iScience. 2021. PMID: 33948557 Free PMC article.
-
Aged Muscle Demonstrates Fiber-Type Adaptations in Response to Mechanical Overload, in the Absence of Myofiber Hypertrophy, Independent of Satellite Cell Abundance.J Gerontol A Biol Sci Med Sci. 2016 Apr;71(4):461-7. doi: 10.1093/gerona/glv033. Epub 2015 Apr 15. J Gerontol A Biol Sci Med Sci. 2016. PMID: 25878030 Free PMC article.
-
Effective fiber hypertrophy in satellite cell-depleted skeletal muscle.Development. 2011 Sep;138(17):3657-66. doi: 10.1242/dev.068858. Development. 2011. PMID: 21828094 Free PMC article.
-
Fusion and beyond: Satellite cell contributions to loading-induced skeletal muscle adaptation.FASEB J. 2021 Oct;35(10):e21893. doi: 10.1096/fj.202101096R. FASEB J. 2021. PMID: 34480776 Free PMC article. Review.
-
Starring or Supporting Role? Satellite Cells and Skeletal Muscle Fiber Size Regulation.Physiology (Bethesda). 2018 Jan 1;33(1):26-38. doi: 10.1152/physiol.00019.2017. Physiology (Bethesda). 2018. PMID: 29212890 Free PMC article. Review.
Cited by
-
miRNA-1 regulation is necessary for mechanical overload-induced muscle hypertrophy in male mice.Physiol Rep. 2025 Jan;13(1):e70166. doi: 10.14814/phy2.70166. Physiol Rep. 2025. PMID: 39761956 Free PMC article.
-
Early satellite cell communication creates a permissive environment for long-term muscle growth.iScience. 2021 Mar 29;24(4):102372. doi: 10.1016/j.isci.2021.102372. eCollection 2021 Apr 23. iScience. 2021. PMID: 33948557 Free PMC article.
-
Extracellular vesicle secretion is tissue-dependent ex vivo and skeletal muscle myofiber extracellular vesicles reach the circulation in vivo.Am J Physiol Cell Physiol. 2022 Feb 1;322(2):C246-C259. doi: 10.1152/ajpcell.00580.2020. Epub 2021 Dec 15. Am J Physiol Cell Physiol. 2022. PMID: 34910603 Free PMC article.
-
Myonuclear transcriptional dynamics in response to exercise following satellite cell depletion.iScience. 2021 Jul 10;24(8):102838. doi: 10.1016/j.isci.2021.102838. eCollection 2021 Aug 20. iScience. 2021. PMID: 34368654 Free PMC article.
-
Mechanical loading of bioengineered skeletal muscle in vitro recapitulates gene expression signatures of resistance exercise in vivo.J Cell Physiol. 2021 Sep;236(9):6534-6547. doi: 10.1002/jcp.30328. Epub 2021 Feb 15. J Cell Physiol. 2021. PMID: 33586196 Free PMC article. Clinical Trial.
References
-
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA.. Pax7 is required for the specification of myogenic satellite cells. Cell 2000;102(6):777–786. - PubMed
-
- Egner IM, Bruusgaard JC, Gundersen K.. Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development 2016;143:2898–2906. - PubMed
-
- McCarthy JJ, Dupont-Versteegden EE, Fry CS, Murach KA, Peterson CA.. Methodological issues limit interpretation of negative effects of satellite cell depletion on adult muscle hypertrophy. Development 2017;144:1363–1365. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
