Inhibition of voltage-gated Na+ currents by eleclazine in rat atrial and ventricular myocytes
- PMID: 32864638
- PMCID: PMC7442036
- DOI: 10.1016/j.hroo.2020.05.006
Inhibition of voltage-gated Na+ currents by eleclazine in rat atrial and ventricular myocytes
Abstract
Background: Atrial-ventricular differences in voltage-gated Na+ currents might be exploited for atrial-selective antiarrhythmic drug action for the suppression of atrial fibrillation without risk of ventricular tachyarrhythmia. Eleclazine (GS-6615) is a putative antiarrhythmic drug with properties similar to the prototypical atrial-selective Na+ channel blocker ranolazine that has been shown to be safe and well tolerated in patients.
Objective: The present study investigated atrial-ventricular differences in the biophysical properties and inhibition by eleclazine of voltage-gated Na+ currents.
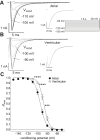
Methods: The fast and late components of whole-cell voltage-gated Na+ currents (respectively, I Na and I NaL) were recorded at room temperature (∼22°C) from rat isolated atrial and ventricular myocytes.
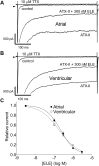
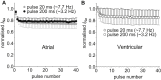
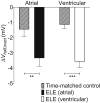
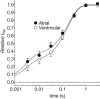
Results: Atrial I Na activated at command potentials ∼5.5 mV more negative and inactivated at conditioning potentials ∼7 mV more negative than ventricular I Na. There was no difference between atrial and ventricular myocytes in the eleclazine inhibition of I NaL activated by 3 nM ATX-II (IC50s ∼200 nM). Eleclazine (10 μM) inhibited I Na in atrial and ventricular myocytes in a use-dependent manner consistent with preferential activated state block. Eleclazine produced voltage-dependent instantaneous inhibition in atrial and ventricular myocytes; it caused a negative shift in voltage of half-maximal inactivation and slowed the recovery of I Na from inactivation in both cell types.
Conclusions: Differences exist between rat atrial and ventricular myocytes in the biophysical properties of I Na. The more negative voltage dependence of I Na activation/inactivation in atrial myocytes underlies differences between the 2 cell types in the voltage dependence of instantaneous inhibition by eleclazine. Eleclazine warrants further investigation as an atrial-selective antiarrhythmic drug.
Keywords: Antiarrhythmic drug; Atrial myocytes; Cardiac regional heterogeneity; INa; INa,Late; Na+ channel blocker; Ventricular myocytes.
© 2020 Heart Rhythm Society. Published by Elsevier Inc.
Figures


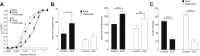
References
-
- Camm A.J., Kirchhof P., Lip G.Y.H. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. - PubMed
-
- Naccarelli G.V., Varker H., Lin J., Schulman K.L. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. - PubMed
-
- Carmeliet E., Vereecke J. 1st ed. Kluwer Academic Publishers; New York: 2002. Cardiac Cellular Electrophysiology.
-
- Lafuente-Lafuente C., Valembois L., Bergmann J.-F., Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Sys Rev. 2015;3:CD005049. - PubMed
-
- Alboni P., Botto G.L., Baldi N. Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Engl J Med. 2004;351:2384–2391. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

