The Interpretation of SARS-CoV-2 Diagnostic Tests
- PMID: 32864639
- PMCID: PMC7441939
- DOI: 10.1016/j.medj.2020.08.001
The Interpretation of SARS-CoV-2 Diagnostic Tests
Abstract
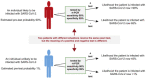
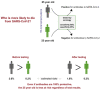
Testing for SARS-CoV-2 has attracted a tremendous amount of attention as a tool to manage the ongoing COVID-19 pandemic. Although diagnostic laboratory testing is used ubiquitously by physicians and encountered regularly by individuals receiving medical care, several aspects of test interpretation are incompletely understood by medical communities and the general population, creating a significant challenge in minimizing the damage caused by disease spread through informed decision making and proper testing utilization. Here, general principles of test interpretation are reviewed and applied to specific examples, such as whether asymptomatic individuals should be tested, what it means to test positive (or negative), and how to interpret tests for "immunity passports." Unexpectedly, the answers seem to run contrary to many of the popular narratives about testing as a tool for managing COVID-19. Although testing is an important and essential part of managing diseases such as COVID-19, improper utilization can have unintended negative consequences.
© 2020 Elsevier Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

