Optimizing Treatment De-Escalation in Head and Neck Cancer: Current and Future Perspectives
- PMID: 32864799
- PMCID: PMC7794179
- DOI: 10.1634/theoncologist.2020-0303
Optimizing Treatment De-Escalation in Head and Neck Cancer: Current and Future Perspectives
Abstract
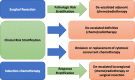
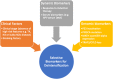
Treatment of locoregionally advanced head and neck squamous cell carcinoma involves a multidisciplinary approach that combines surgery, radiotherapy, and systemic therapy. These curative strategies are associated with significant acute and long-term toxicities. With the emergence of human papillomavirus (HPV) as an etiologic factor associated primarily with oropharyngeal squamous cell carcinoma, higher cure rates juxtaposed with substantial treatment-related morbidity and mortality has led to interest in de-escalated therapeutic strategies, with the goal of optimizing oncologic outcomes while reducing treatment-related toxicity. Currently explored strategies include replacing, reducing, or omitting cytotoxic chemotherapy; reducing dose or volume of radiotherapy; and incorporation of less-invasive surgical approaches. Potential biomarkers to select patients for treatment de-escalation include clinical risk stratification, adjuvant de-escalation based on pathologic features, response to induction therapy, and molecular markers. The optimal patient selection and de-escalation strategy is critically important in the evolving treatment of locoregional head and neck cancer. Recently, two large phase III trials, RTOG 1016 and De-ESCALaTE, failed to de-escalate treatment in HPV-associated head and neck cancer by demonstrating inferior outcomes by replacing cisplatin with cetuximab in combination with radiation. This serves as a cautionary tale in the future design of de-escalation trials in this patient population, which will need to leverage toxicity and efficacy endpoints. Our review summarizes completed and ongoing de-escalation trials in head and neck cancer, with particular emphasis on biomarkers for patient selection and clinical trial design. IMPLICATIONS FOR PRACTICE: The toxicity associated with standard multimodality treatment for head and neck cancer underscores the need to seek less-intensive therapies with a reduced long-term symptom burden through de-escalated treatment paradigms that minimize toxicity while maintaining oncologic control in appropriately selected patients. Controversy regarding the optimal de-escalation strategy and criteria for patient selection for de-escalated therapy has led to multiple parallel strategies undergoing clinical investigation. Well-designed trials that optimize multimodal strategies are needed. Given the absence of positive randomized trials testing de-escalated therapy to date, practicing oncologists should exercise caution and administer established standard-of-care therapy outside the context of a clinical trial.
Keywords: Head and neck cancer; Human papillomavirus; Multimodality therapy; Treatment de-escalation.
© 2020 AlphaMed Press.
Conflict of interest statement
Figures
References
-
- Nguyen‐Tan PF, Zhang Q, Ang KK et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: Long‐term report of efficacy and toxicity. J Clin Oncol 2014;32:3858–3866. - PMC - PubMed
-
- Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical