Diagnosis of SARS-CoV-2 by RT-PCR Using Different Sample Sources: Review of the Literature
- PMID: 32865458
- PMCID: PMC7459180
- DOI: 10.1177/0145561320953231
Diagnosis of SARS-CoV-2 by RT-PCR Using Different Sample Sources: Review of the Literature
Abstract
Objective: The most widely used diagnostic technique for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is real-time reverse transcriptase-polymerase chain reaction (RT-PCR). It can be done on different samples: nasopharyngeal swabs (NPS) or oropharyngeal swabs (OPS), and self-collected saliva. However, negative findings do not rule out infection.
Methods: A review was conceived to discuss advantages and limitations of the available diagnostic modalities for nonserologic diagnosis of SARS-CoV-2 based on RT-PCR; the article also proposes some practical suggestions to improve diagnostic reliability.
Results: A total of 16 papers (corresponding to 452 patients) of the 56 initially identified were included. Most of the papers describe findings from different samples obtained in limited case series; comparative studies are missing.
Conclusions: Diagnostic accuracy of NPS and OPS is suboptimal and the risk of contaminated aerosol dispersal is not negligible. The SARS-CoV-2 RNA can be found in self-collected saliva specimens of many infected patients within 7 to 10 days after symptom onset. There is an urgent need for comparative trials to define the diagnostic modality of choice. Adequate education and training of health care personnel is mandatory.
Keywords: COVID-19; emergency; infection; nasopharynx; swab.
Conflict of interest statement
Figures
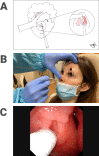
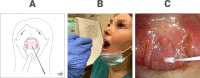
References
-
- World Health Organization. Coronavirus Disease 2019 (COVID-19). Situation Report-120. Updated June 10, 2020. Accessed June 11, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2....
-
- Centers for Disease Control and Prevention. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19). Updated July 8, 2020. Accessed April 16, 2020. https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specim...
-
- Lippi G, Simundic AM, Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19). Clin Chem Lab Med. 2020;58(7):1070–1076. - PubMed
-
- Food and Drug Administration. New-York SARS-CoV-2. Real-Time RT-PCR Diagnostic Panel-February 29. Updated March 15, 2020. Accessed March 9, 2020. https://www.fda.gov/media/135662/download
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

