Protein Kinase C Alpha (PKCα) overexpression leads to a better response to retinoid acid therapy through Retinoic Acid Receptor Beta (RARβ) activation in mammary cancer cells
- PMID: 32865619
- PMCID: PMC11804370
- DOI: 10.1007/s00432-020-03368-7
Protein Kinase C Alpha (PKCα) overexpression leads to a better response to retinoid acid therapy through Retinoic Acid Receptor Beta (RARβ) activation in mammary cancer cells
Abstract
Purpose: Retinoids have proved to be effective for hematologic malignancies treatment but till nowadays, their use as single agent for the solid tumor's management is still controversial. All-trans retinoic acid (ATRA), the main active metabolite of vitamin A, exerts non-genomic interactions with different members of the protein kinase C (PKC) family, recognized modulators of different tumor progression pathways. To determine whether a group of patients could become benefited employing a retinoid therapy, in this study we have evaluated whether PKCα expression (a poor prognosis marker in breast cancer) could sensitizes mammary cells to ATRA treatment.
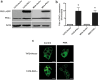
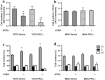
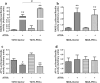
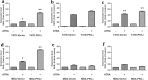
Methods: PKCα overexpression was achieved by stable transfection and confirmed by western blot. Transfected PKC functionality was determined by nuclear translocation-induction and confocal microscopy. In vitro proliferation was evaluated by cell counting and cell cycle distribution was analyzed by flow cytometry. In vivo studies were performed to evaluate orthotopic tumor growth and experimental lung colonization. Retinoic acid response elements (RARE) and AP1 sites-dependent activity was studied by gene reporter assays and retinoic acid receptors (RARs) were measured by RT-qPCR.
Results: Our findings suggest that high PKCα levels improve the differentiation response to ATRA in a RAR signaling-dependent manner. Moreover, RARβ expression appears to be critical to induce ATRA sensitization, throughout AP1 trans-repression.
Conclusion: Here we propose that retinoids could lead a highly personalized anticancer treatment, bringing benefits to patients with aggressive breast tumors resulting from high PKCα expression but, an adequate expression of the RARβ receptor is required to ensure the effect on this process.
Keywords: ATRA; Metastasis dissemination; PKCα; Proliferation; Tumor growth.
Conflict of interest statement
The authors read and approved the manuscript and declare that they have no competing interests.
Figures

Similar articles
-
Retinoic Acid Receptor β: A Potential Therapeutic Target in Retinoic Acid Treatment of Endometrial Cancer.Int J Gynecol Cancer. 2017 May;27(4):643-650. doi: 10.1097/IGC.0000000000000995. Int J Gynecol Cancer. 2017. PMID: 28375930
-
Notch3 enhances the synergistic effect of all-trans retinoic acid and calcipotriol in pancreatic stellate cell activation.J Transl Med. 2025 Jun 22;23(1):694. doi: 10.1186/s12967-025-06666-1. J Transl Med. 2025. PMID: 40545532 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Additive effects of PI3-kinase and MAPK activities on NB4 cell granulocyte differentiation: potential role of phosphatidylinositol 3-kinase gamma.J Cancer Res Clin Oncol. 2008 Aug;134(8):861-72. doi: 10.1007/s00432-008-0356-8. Epub 2008 Feb 21. J Cancer Res Clin Oncol. 2008. PMID: 18288489 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
Cited by
-
Antineoplastic activity of products derived from cellulose-containing materials: levoglucosenone and structurally-related derivatives as new alternatives for breast cancer treatment.Invest New Drugs. 2022 Feb;40(1):30-41. doi: 10.1007/s10637-021-01167-6. Epub 2021 Sep 3. Invest New Drugs. 2022. PMID: 34478029
-
Mucin 4 expression is associated with metastasis in triple-negative breast cancer and can be tackled by soluble TNF blockade, improving immunotherapy outcome.Transl Oncol. 2025 Apr;54:102325. doi: 10.1016/j.tranon.2025.102325. Epub 2025 Feb 22. Transl Oncol. 2025. PMID: 39987883 Free PMC article.
-
Involvement of hedgehog signaling in all-trans retinoic acid-mediated suppression of colon cancer.Am J Transl Res. 2022 Sep 15;14(9):6536-6549. eCollection 2022. Am J Transl Res. 2022. PMID: 36247302 Free PMC article.
-
CRISPR/Cas9-mediated knockout strategies for enhancing immunotherapy in breast cancer.Naunyn Schmiedebergs Arch Pharmacol. 2024 Nov;397(11):8561-8601. doi: 10.1007/s00210-024-03208-2. Epub 2024 Jun 22. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38907847 Review.
-
The Protective Anticancer Effect of Natural Lycopene Supercritical CO2 Watermelon Extracts in Adenocarcinoma Lung Cancer Cells.Antioxidants (Basel). 2022 Jun 11;11(6):1150. doi: 10.3390/antiox11061150. Antioxidants (Basel). 2022. PMID: 35740047 Free PMC article.
References
-
- Berardi DE, Bessone MI, Motter A, de Kier B, Joffe ED, Urtreger AJ, Todaro LB (2015) Involvement of protein kinase C alpha and delta activities on the induction of the retinoic acid system in mammary cancer cells. Mol Carcinog 54:1110–1121. 10.1002/mc.22181 - PubMed
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA. Cancer J Clin 68:394–424. 10.3322/caac.21492 - PubMed
-
- Cameron AJ, Procyk KJ, Leitges M, Parker PJ (2008) PKC alpha protein but not kinase activity is critical for glioma cell proliferation and survival. Int J Cancer 123:769–779. 10.1002/ijc.23560 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

