Tolerance to Opioid-Induced Respiratory Depression in Chronic High-Dose Opioid Users: A Model-Based Comparison With Opioid-Naïve Individuals
- PMID: 32865832
- PMCID: PMC7983936
- DOI: 10.1002/cpt.2027
Tolerance to Opioid-Induced Respiratory Depression in Chronic High-Dose Opioid Users: A Model-Based Comparison With Opioid-Naïve Individuals
Abstract
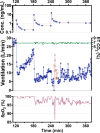
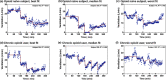
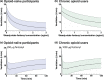
Chronic opioid consumption is associated with addiction, physical dependence, and tolerance. Tolerance results in dose escalation to maintain the desired opioid effect. Intake of high-dose or potent opioids may cause life-threatening respiratory depression, an effect that may be reduced by tolerance. We performed a pharmacokinetic-pharmacodynamic analysis of the respiratory effects of fentanyl in chronic opioid users and opioid-naïve subjects to quantify tolerance to respiratory depression. Fourteen opioid-naïve individuals and eight chronic opioid users received escalating doses of intravenous fentanyl (opioid-naïve subjects: 75-350 µg/70 kg; chronic users: 250-700 µg/70 kg). Isohypercapnic ventilation was measured and the fentanyl plasma concentration-ventilation data were analyzed using nonlinear mixed-effects modeling. Apneic events occurred in opioid-naïve subjects after a cumulative fentanyl dose (per 70 kg) of 225 (n = 3) and 475 µg (n = 6), and in 7 chronic opioid users after a cumulative dose of 600 (n = 2), 1,100 (n = 2), and 1,800 µg (n = 3). The time course of fentanyl's respiratory depressant effect was characterized using a biophase equilibration model in combination with an inhibitory maximum effect (Emax ) model. Differences in tolerance between populations were successfully modeled. The effect-site concentration causing 50% ventilatory depression, was 0.42 ± 0.07 ng/mL in opioid-naïve subjects and 1.82 ± 0.39 ng/mL in chronic opioid users, indicative of a 4.3-fold sensitivity difference. Despite higher tolerance to fentanyl-induced respiratory depression, apnea still occurred in the opioid-tolerant population indicative of the potential danger of high-dose opioids in causing life-threatening respiratory depression in all individuals, opioid-naïve and opioid-tolerant.
© 2020 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
R.L.D. and C.M.L. are employees of Indivior and declare no other competing interests. All other authors declared no competing interests for this work.
Figures


Similar articles
-
Preoperative "fentanyl challenge" as a tool to estimate postoperative opioid dosing in chronic opioid-consuming patients.Anesth Analg. 2005 Aug;101(2):389-395. doi: 10.1213/01.ANE.0000156563.25878.19. Anesth Analg. 2005. PMID: 16037150
-
Effect of sustained high buprenorphine plasma concentrations on fentanyl-induced respiratory depression: A placebo-controlled crossover study in healthy volunteers and opioid-tolerant patients.PLoS One. 2022 Jan 27;17(1):e0256752. doi: 10.1371/journal.pone.0256752. eCollection 2022. PLoS One. 2022. PMID: 35085249 Free PMC article. Clinical Trial.
-
Mechanism-based PK/PD modeling of the respiratory depressant effect of buprenorphine and fentanyl in healthy volunteers.Clin Pharmacol Ther. 2007 Jan;81(1):50-8. doi: 10.1038/sj.clpt.6100025. Clin Pharmacol Ther. 2007. PMID: 17185999 Clinical Trial.
-
Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone).Pain Pract. 2008 Jul-Aug;8(4):287-313. doi: 10.1111/j.1533-2500.2008.00204.x. Epub 2008 May 23. Pain Pract. 2008. PMID: 18503626
-
Fentanyl buccal tablet: in breakthrough pain in opioid-tolerant patients with cancer.Drugs. 2006;66(18):2387-93; discussion 2394-5. doi: 10.2165/00003495-200666180-00013. Drugs. 2006. PMID: 17181383 Review.
Cited by
-
Advances in attenuating opioid-induced respiratory depression: A narrative review.Medicine (Baltimore). 2024 Jul 19;103(29):e38837. doi: 10.1097/MD.0000000000038837. Medicine (Baltimore). 2024. PMID: 39029082 Free PMC article. Review.
-
Mechanism-based organization of neural networks to emulate systems biology and pharmacology models.Sci Rep. 2024 May 27;14(1):12082. doi: 10.1038/s41598-024-59378-9. Sci Rep. 2024. PMID: 38802422 Free PMC article.
-
Respiratory Depression Associated with Opioids: A Narrative Review.Curr Treat Options Oncol. 2024 Nov;25(11):1438-1450. doi: 10.1007/s11864-024-01274-5. Epub 2024 Oct 21. Curr Treat Options Oncol. 2024. PMID: 39432171 Review.
-
Time Course of Reversal of Fentanyl-Induced Respiratory Depression in Healthy Subjects by Intramuscular Nalmefene and Intramuscular and Intranasal Naloxone.J Clin Pharmacol. 2025 Feb;65(2):206-216. doi: 10.1002/jcph.6132. Epub 2024 Sep 30. J Clin Pharmacol. 2025. PMID: 39347921 Free PMC article. Clinical Trial.
-
Oxygen fluctuations in the brain and periphery induced by intravenous fentanyl: effects of dose and drug experience.J Neurophysiol. 2024 Aug 1;132(2):322-334. doi: 10.1152/jn.00177.2024. Epub 2024 Jun 12. J Neurophysiol. 2024. PMID: 38863429 Free PMC article.
References
-
- Freye, E. & Latasch, L. Development of opioid tolerance – molecular mechanisms and clinical consequences. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 38, 14–26 (2003). - PubMed
-
- Colvin, L.A. , Bull, F. & Hales, T.G. Perioperative opioid analgesia‐when is enough too much? A review of opioid‐induced tolerance and hyperalgesia. Lancet 393, 1558–1568 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

