LMTK3 promotes tumorigenesis in bladder cancer via the ERK/MAPK pathway
- PMID: 32865871
- PMCID: PMC7530379
- DOI: 10.1002/2211-5463.12964
LMTK3 promotes tumorigenesis in bladder cancer via the ERK/MAPK pathway
Erratum in
-
Corrigendum to: LMTK3 promotes tumorigenesis in bladder cancer via the ERK/MAPK pathway.FEBS Open Bio. 2021 Apr;11(4):1277. doi: 10.1002/2211-5463.13116. Epub 2021 Mar 2. FEBS Open Bio. 2021. PMID: 33794072 Free PMC article. No abstract available.
Abstract
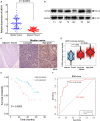
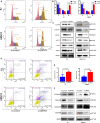
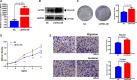
Lemur tyrosine kinase 3 (LMTK3) is a key member of the serine-threonine tyrosine kinase family. It plays an important role in breast cancer tumorigenesis and progression. However, its biological role in bladder cancer remains elusive. In this study, we demonstrated that LMTK3 was overexpressed in bladder cancer and was positively correlated with bladder cancer malignancy. High LMTK3 expression predicted poor overall survival. Knockdown of LMTK3 in bladder cancer cells triggered cell-cycle arrest at G2/M phase, suppressed cell growth, and induced cell apoptosis in bladder cancer cells. Furthermore, Transwell assays revealed that reduction of LMTK3 decreased cell migration by regulating the epithelial-to-mesenchymal transition pathway. Conversely, LKTM3 overexpression was shown to promote proliferation and migration of bladder cancer cells. We assessed phosphorylation of MEK and ERK1/2 in bladder cancer cells depleted of LMTK3 and demonstrated a reduced phosphorylation status compared with the control group. Using an MAPK signaling-specific inhibitor, U0126, we could rescue the promotion of proliferation and viability in LMTK3-overexpressing cells. In conclusion, we extend the status of LMTK3 as an oncogene in bladder cancer and provide evidence for its function via the activation of the ERK/MAPK pathway. Thus, targeting LMTK3 may hold potential as a diagnostic and prognostic biomarker and as a possible future treatment for bladder cancer.
Keywords: biomarker; bladder cancer; lemur tyrosine kinase-3; weighted gene coexpression network analysis.
© 2020 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Decreased TRPM7 inhibits activities and induces apoptosis of bladder cancer cells via ERK1/2 pathway.Oncotarget. 2016 Nov 8;7(45):72941-72960. doi: 10.18632/oncotarget.12146. Oncotarget. 2016. PMID: 27662662 Free PMC article.
-
Lemur tyrosine kinase-3 (LMTK3) induces chemoresistance to cetuximab in colorectal cancer via the ERK/MAPK pathway.Bioengineered. 2021 Dec;12(1):6594-6605. doi: 10.1080/21655979.2021.1974655. Bioengineered. 2021. PMID: 34516351 Free PMC article.
-
Downregulation of LAPTM5 suppresses cell proliferation and viability inducing cell cycle arrest at G0/G1 phase of bladder cancer cells.Int J Oncol. 2017 Jan;50(1):263-271. doi: 10.3892/ijo.2016.3788. Epub 2016 Dec 5. Int J Oncol. 2017. PMID: 27922670
-
The multifaceted role of lemur tyrosine kinase 3 in health and disease.Open Biol. 2021 Sep;11(9):210218. doi: 10.1098/rsob.210218. Epub 2021 Sep 29. Open Biol. 2021. PMID: 34582708 Free PMC article. Review.
-
The context-specific roles of urea cycle enzymes in tumorigenesis.Mol Cell. 2021 Sep 16;81(18):3749-3759. doi: 10.1016/j.molcel.2021.08.005. Epub 2021 Aug 31. Mol Cell. 2021. PMID: 34469752 Review.
Cited by
-
Lemur Tyrosine Kinases and Prostate Cancer: A Literature Review.Int J Mol Sci. 2021 May 21;22(11):5453. doi: 10.3390/ijms22115453. Int J Mol Sci. 2021. PMID: 34064250 Free PMC article. Review.
-
HAPLN3 inhibits apoptosis and promotes EMT of clear cell renal cell carcinoma via ERK and Bcl-2 signal pathways.J Cancer Res Clin Oncol. 2023 Jan;149(1):79-90. doi: 10.1007/s00432-022-04421-3. Epub 2022 Nov 14. J Cancer Res Clin Oncol. 2023. PMID: 36374334 Free PMC article.
-
Corrigendum to: LMTK3 promotes tumorigenesis in bladder cancer via the ERK/MAPK pathway.FEBS Open Bio. 2021 Apr;11(4):1277. doi: 10.1002/2211-5463.13116. Epub 2021 Mar 2. FEBS Open Bio. 2021. PMID: 33794072 Free PMC article. No abstract available.
-
The lemur tail kinase family in neuronal function and disfunction in neurodegenerative diseases.Cell Mol Life Sci. 2024 Nov 9;81(1):447. doi: 10.1007/s00018-024-05480-0. Cell Mol Life Sci. 2024. PMID: 39520508 Free PMC article. Review.
-
NCAPH promotes cell proliferation and inhibits cell apoptosis of bladder cancer cells through MEK/ERK signaling pathway.Cell Cycle. 2022 Feb;21(4):427-438. doi: 10.1080/15384101.2021.2021050. Epub 2022 Jan 2. Cell Cycle. 2022. PMID: 34974790 Free PMC article.
References
-
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ and He J. (2016) Cancer statistics in China, 2015. CA: a Cancer J Clin 66, 115‐32. - PubMed
-
- Sun P, Sun X, Zhao W, Ren M, Zhang C, Wang Z and Xu W (2017) Lemur tyrosine kinase‐3 suppresses growth of prostate cancer via the AKT and MAPK signaling pathways. Cell Physiol Biochem 42, 2582–2592. - PubMed
-
- Asano T, Sato S, Yoshimoto N, Endo Y, Hato Y, Dong Y, Takahashi S, Fujii Y and Toyama T (2014) High expression of LMTK3 is an independent factor indicating a poor prognosis in estrogen receptor alpha‐positive breast cancer patients. Jpn J Clin Oncol 44, 889–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

