Accurate Identification of Isomeric Glycans by Trapped Ion Mobility Spectrometry-Electronic Excitation Dissociation Tandem Mass Spectrometry
- PMID: 32865981
- PMCID: PMC8111820
- DOI: 10.1021/acs.analchem.0c02374
Accurate Identification of Isomeric Glycans by Trapped Ion Mobility Spectrometry-Electronic Excitation Dissociation Tandem Mass Spectrometry
Abstract
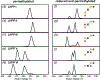
Ion mobility-mass spectrometry (IM-MS) has become a powerful tool for glycan structural characterization due to its ability to separate isomers and provide collision cross section (CCS) values that facilitate structural assignment. However, IM-based isomer analysis may be complicated by the presence of multiple gas-phase conformations of a single structure that not only increases difficulty in isomer separation but can also introduce the possibility for misinterpretation of conformers as isomers. Here, the ion mobility behavior of several sets of isomeric glycans, analyzed as their permethylated derivatives, in both nonreduced and reduced forms, was investigated by gated-trapped ion mobility spectrometry (G-TIMS). Notably, reducing-end reduction, commonly performed to remove anomerism-induced chromatographic peak splitting, did not eliminate the conformational heterogeneity of permethylated glycans in the gas phase. At a mobility resolving power of ∼100, 14 out of 22 structures showed more than one conformation. These results highlight the need to use IMS devices with high mobility resolving power for better separation of isomers and to acquire additional structural information that can differentiate isomers from conformers. Online electronic excitation dissociation (EED) MS/MS analysis of isomeric glycan mixtures following G-TIMS separation showed that EED can generate isomer-specific fragments while producing nearly identical tandem mass spectra for conformers, thus allowing confident identification of isomers with minimal evidence of any ambiguity resulting from the presence of conformers. G-TIMS EED MS/MS analysis of N-linked glycans released from ovalbumin revealed that several mobility features previously thought to arise from isomeric structures were conformers of a single structure. Finally, analysis of ovalbumin N-glycans from different sources showed that the G-TIMS EED MS/MS approach can accurately determine the batch-to-batch variations in glycosylation profiles at the isomer level, with confident assignment of each isomeric structure.
Figures

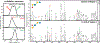


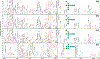
Similar articles
-
Separation and Identification of Isomeric Glycans by Selected Accumulation-Trapped Ion Mobility Spectrometry-Electron Activated Dissociation Tandem Mass Spectrometry.Anal Chem. 2016 Apr 5;88(7):3440-3. doi: 10.1021/acs.analchem.6b00041. Epub 2016 Mar 14. Anal Chem. 2016. PMID: 26959868 Free PMC article.
-
Characterization of Isomeric Glycans by Reversed Phase Liquid Chromatography-Electronic Excitation Dissociation Tandem Mass Spectrometry.J Am Soc Mass Spectrom. 2018 Jun;29(6):1295-1307. doi: 10.1007/s13361-018-1943-9. Epub 2018 Apr 13. J Am Soc Mass Spectrom. 2018. PMID: 29654534 Free PMC article.
-
Collision Cross Sections and Ion Mobility Separation of Fragment Ions from Complex N-Glycans.J Am Soc Mass Spectrom. 2018 Jun;29(6):1250-1261. doi: 10.1007/s13361-018-1930-1. Epub 2018 Apr 19. J Am Soc Mass Spectrom. 2018. PMID: 29675741
-
Applications of ion mobility mass spectrometry for high throughput, high resolution glycan analysis.Biochim Biophys Acta. 2016 Aug;1860(8):1688-709. doi: 10.1016/j.bbagen.2016.02.003. Epub 2016 Feb 22. Biochim Biophys Acta. 2016. PMID: 26854953 Review.
-
Analysis of N- and O-linked site-specific glycosylation by ion mobility mass spectrometry: State of the art and future directions.Proteomics. 2024 Jun;24(12-13):e2300281. doi: 10.1002/pmic.202300281. Epub 2024 Jan 3. Proteomics. 2024. PMID: 38171879 Review.
Cited by
-
Structural O-Glycoform Heterogeneity of the SARS-CoV-2 Spike Protein Receptor-Binding Domain Revealed by Top-Down Mass Spectrometry.J Am Chem Soc. 2021 Aug 11;143(31):12014-12024. doi: 10.1021/jacs.1c02713. Epub 2021 Jul 30. J Am Chem Soc. 2021. PMID: 34328324 Free PMC article.
-
Rapid structural characterization of human milk oligosaccharides and distinction of their isomers using trapped ion mobility spectrometry time-of-flight mass spectrometry.J Mass Spectrom. 2022 Oct;57(10):e4885. doi: 10.1002/jms.4885. J Mass Spectrom. 2022. PMID: 36199270 Free PMC article.
-
Potential of ion mobility mass spectrometry in cellulose ether analysis: substitution pattern of hydroxyethyl celluloses.Anal Bioanal Chem. 2024 Apr;416(10):2527-2539. doi: 10.1007/s00216-024-05224-w. Epub 2024 Mar 4. Anal Bioanal Chem. 2024. PMID: 38436692 Free PMC article.
-
Maximizing Glycoproteomics Results through an Integrated Parallel Accumulation Serial Fragmentation Workflow.Anal Chem. 2024 Jun 4;96(22):8956-8964. doi: 10.1021/acs.analchem.3c05874. Epub 2024 May 22. Anal Chem. 2024. PMID: 38776126 Free PMC article.
-
High-Resolution N-Glycan MALDI Mass Spectrometry Imaging of Subchondral Bone Tissue Microarrays in Patients with Knee Osteoarthritis.Anal Chem. 2023 Aug 29;95(34):12640-12647. doi: 10.1021/acs.analchem.3c00348. Epub 2023 Aug 15. Anal Chem. 2023. PMID: 37583288 Free PMC article.
References
-
- Mancera-Arteu M; Giménez E; Barbosa J; Sanz-Nebot V Identification and characterization of isomeric N-glycans of human alfa-acid-glycoprotein by stable isotope labelling and ZIC-HILIC-MS in combination with exoglycosidase digestion Anal. Chim. Acta 2016, 940, 92–103. - PubMed
-
- Zhao J; Li S; Li C; Wu S-L; Xu W; Chen Y; Shameem M; Richardson D; Li H Identification of low abundant isomeric N-glycan structures in biological therapeutics by LC/MS Anal. Chem 2016, 88, 7049–7059. - PubMed
-
- Mancera-Arteu M; Giménez E; Barbosa J; Peracaula R; Sanz-Nebot V Zwitterionic-hydrophilic interaction capillary liquid chromatography coupled to tandem mass spectrometry for the characterization of human alpha-acid-glycoprotein N-glycan isomers Anal. Chim. Acta 2017, 991, 76–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

