Site-Selective RNA Functionalization via DNA-Induced Structure
- PMID: 32865995
- PMCID: PMC7962339
- DOI: 10.1021/jacs.0c06824
Site-Selective RNA Functionalization via DNA-Induced Structure
Abstract
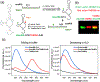
Methods for RNA functionalization at specific sites are in high demand but remain a challenge, particularly for RNAs produced by transcription rather than by total synthesis. Recent studies have described acylimidazole reagents that react in high yields at 2'-OH groups stochastically at nonbase-paired regions, covering much of the RNA in scattered acyl esters. Localized reactions, if possible, could prove useful in many applications, providing functional handles at specific sites and sequences of the biopolymer. Here, we describe a DNA-directed strategy for in vitro functionalization of RNA at site-localized 2'-OH groups. The method, RNA Acylation at Induced Loops (RAIL), utilizes complementary helper DNA oligonucleotides that expose gaps or loops at selected positions while protecting the remainder in DNA-RNA duplexes. Reaction with an acylimidazole reagent is then carried out, providing high yields of 2'-OH conjugation at predetermined sites. Experiments reveal optimal helper oligodeoxynucleotide designs and conditions for the reaction, and tests of the approach are carried out to control localized ribozyme activities and to label RNAs with dual-color fluorescent dyes. The RAIL approach offers a simple and novel strategy for site-selective labeling and control of RNAs, potentially of any length and origin.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Chemical diversity of reagents that modify RNA 2'-OH in water: a review.Chem Sci. 2024 Sep 12;15(39):15968-82. doi: 10.1039/d4sc05317f. Online ahead of print. Chem Sci. 2024. PMID: 39309104 Free PMC article. Review.
-
DNA Tiling Enables Precise Acylation-Based Labeling and Control of mRNA.Angew Chem Int Ed Engl. 2021 Dec 13;60(51):26798-26805. doi: 10.1002/anie.202112106. Epub 2021 Nov 16. Angew Chem Int Ed Engl. 2021. PMID: 34624169 Free PMC article.
-
Second-Generation Chiral Amino Acid Derivatives Afford High Stereoselectivity and Stability in Aqueous RNA Acylation.J Org Chem. 2024 Jun 7;89(11):8055-8063. doi: 10.1021/acs.joc.4c00686. Epub 2024 May 29. J Org Chem. 2024. PMID: 38809698 Free PMC article.
-
Diverse Reagent Scaffolds Provide Differential Selectivity of 2'-OH Acylation in RNA.J Am Chem Soc. 2023 Jan 11;145(1):143-151. doi: 10.1021/jacs.2c09040. Epub 2022 Dec 21. J Am Chem Soc. 2023. PMID: 36542611
-
Click nucleic acid ligation: applications in biology and nanotechnology.Acc Chem Res. 2012 Aug 21;45(8):1258-67. doi: 10.1021/ar200321n. Epub 2012 Mar 22. Acc Chem Res. 2012. PMID: 22439702 Free PMC article. Review.
Cited by
-
Strategies for Covalent Labeling of Long RNAs.Chembiochem. 2021 Oct 1;22(19):2826-2847. doi: 10.1002/cbic.202100161. Epub 2021 Jun 17. Chembiochem. 2021. PMID: 34043861 Free PMC article. Review.
-
DNAzyme-Catalyzed Site-Specific N-Acylation of DNA Oligonucleotide Nucleobases.Angew Chem Int Ed Engl. 2024 Feb 12;63(7):e202317565. doi: 10.1002/anie.202317565. Epub 2024 Jan 11. Angew Chem Int Ed Engl. 2024. PMID: 38157448 Free PMC article.
-
RNA-Polymer Hybrids via Direct and Site-Selective Acylation with the ATRP Initiator and Photoinduced Polymerization.J Am Chem Soc. 2023 Jul 5;145(26):14435-14445. doi: 10.1021/jacs.3c03757. Epub 2023 Jun 26. J Am Chem Soc. 2023. PMID: 37357749 Free PMC article.
-
Chemical diversity of reagents that modify RNA 2'-OH in water: a review.Chem Sci. 2024 Sep 12;15(39):15968-82. doi: 10.1039/d4sc05317f. Online ahead of print. Chem Sci. 2024. PMID: 39309104 Free PMC article. Review.
-
Conjugation of RNA via 2'-OH acylation: Mechanisms determining nucleotide reactivity.Chem Commun (Camb). 2022 Mar 15;58(22):3693-3696. doi: 10.1039/d2cc00660j. Chem Commun (Camb). 2022. PMID: 35226025 Free PMC article.
References
-
- He C, Grand Challenge Commentary: RNA epigenetics? Nat. Chem. Biol 2010, 6 (12), 863–865. - PubMed
-
- Cech Thomas R.; Steitz Joan A., The Noncoding RNA Revolution-Trashing Old Rules to Forge New Ones. Cell 2014, 157 (1), 77–94. - PubMed
-
- Pichon X; Lagha M; Mueller F; Bertrand E, A Growing Toolbox to Image Gene Expression in Single Cells: Sensitive Approaches for Demanding Challenges. Mol. Cell 2018, 71 (3), 468–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

