Safety, Immunogenicity, and Hemostatic Efficacy of Nonacog Gamma in Patients With Severe or Moderately Severe Hemophilia B: A Continuation Study
- PMID: 32866032
- PMCID: PMC7469725
- DOI: 10.1177/1076029620950836
Safety, Immunogenicity, and Hemostatic Efficacy of Nonacog Gamma in Patients With Severe or Moderately Severe Hemophilia B: A Continuation Study
Abstract
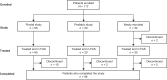
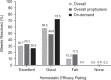
This phase 3, prospective, open-label, multicenter, continuation study (NCT01286779) investigated the use of a recombinant factor IX (FIX), nonacog gamma (BAX 326, RIXUBIS®) in patients with severe or moderately severe hemophilia B. The study population included 85 patients transitioning from a phase 1/3 pivotal study (NCT01174446), a pediatric study (NCT01488994), and 30 newly recruited patients, naïve to nonacog gamma. Patients received nonacog gamma as prophylaxis treatment (standard, modified or PK-tailored) or on-demand, as determined by the investigator. Treatment was assessed for safety, immunogenicity, hemostatic efficacy and consumption. In this study, after ≥100 exposure days, nonacog gamma resulted in no treatment-related serious adverse events, and no patients developed inhibitory antibodies to FIX. Nonacog gamma was efficacious at controlling bleeding episodes, with an 89.1% overall hemostatic efficacy rating of excellent or good, and 56% of bleeds resolved with one infusion. The annualized bleeding rate was considerably lower during prophylactic treatment (median ABR of 1.3 in 108 patients) than during on-demand treatment (median ABR of 16.5 in 13 patients). These results show that in previously treated patients and nonacog gamma-naïve patients, long-term use of nonacog gamma had acceptable safety and tolerability, and was efficacious as a prophylactic treatment for the management of bleeding episodes.NCT01286779, EudraCT: 2010-022726-33.
Keywords: continuation study; factor IX; hemophilia B; hemostatic efficacy; nonacog gamma; safety.
Conflict of interest statement
Figures

References
-
- Srivastava A, Brewer A, Mauser-Bunschoten E, et al. Treatment Guidelines Working Group on Behalf of the World Federation of Hemophilia. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47. - PubMed
-
- Key NS. Inhibitors in congenital coagulation disorders. Br J Haematol. 2004;127(4):379–391. - PubMed
-
- US Food and Drug Administration. RIXUBIS. Product information. Published 2014 Accessed October 1, 2019 http://www.fda.gov/vaccines-blood-biologics/approved-blood-products/rixubis.
-
- European Medicines Agency. RIXUBIS. Summary of product characteristics. Accessed October 1, 2019 https://www.ema.europa.eu/documents/product-information/rixubis-epar-pro....
-
- Windyga J, Lissitchkov T, Stasyshyn O, et al. Pharmacokinetics, efficacy and safety of BAX326, a novel recombinant factor IX: a prospective, controlled, multicenter phase I/III study in previously treated patients with severe (FIX level <1%) or moderately severe (FIX level ≤2%) haemophilia B. Haemophilia. 2014;20(1):15–24. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

