Uncontrolled Innate and Impaired Adaptive Immune Responses in Patients with COVID-19 Acute Respiratory Distress Syndrome
- PMID: 32866033
- PMCID: PMC7706149
- DOI: 10.1164/rccm.202005-1885OC
Uncontrolled Innate and Impaired Adaptive Immune Responses in Patients with COVID-19 Acute Respiratory Distress Syndrome
Abstract
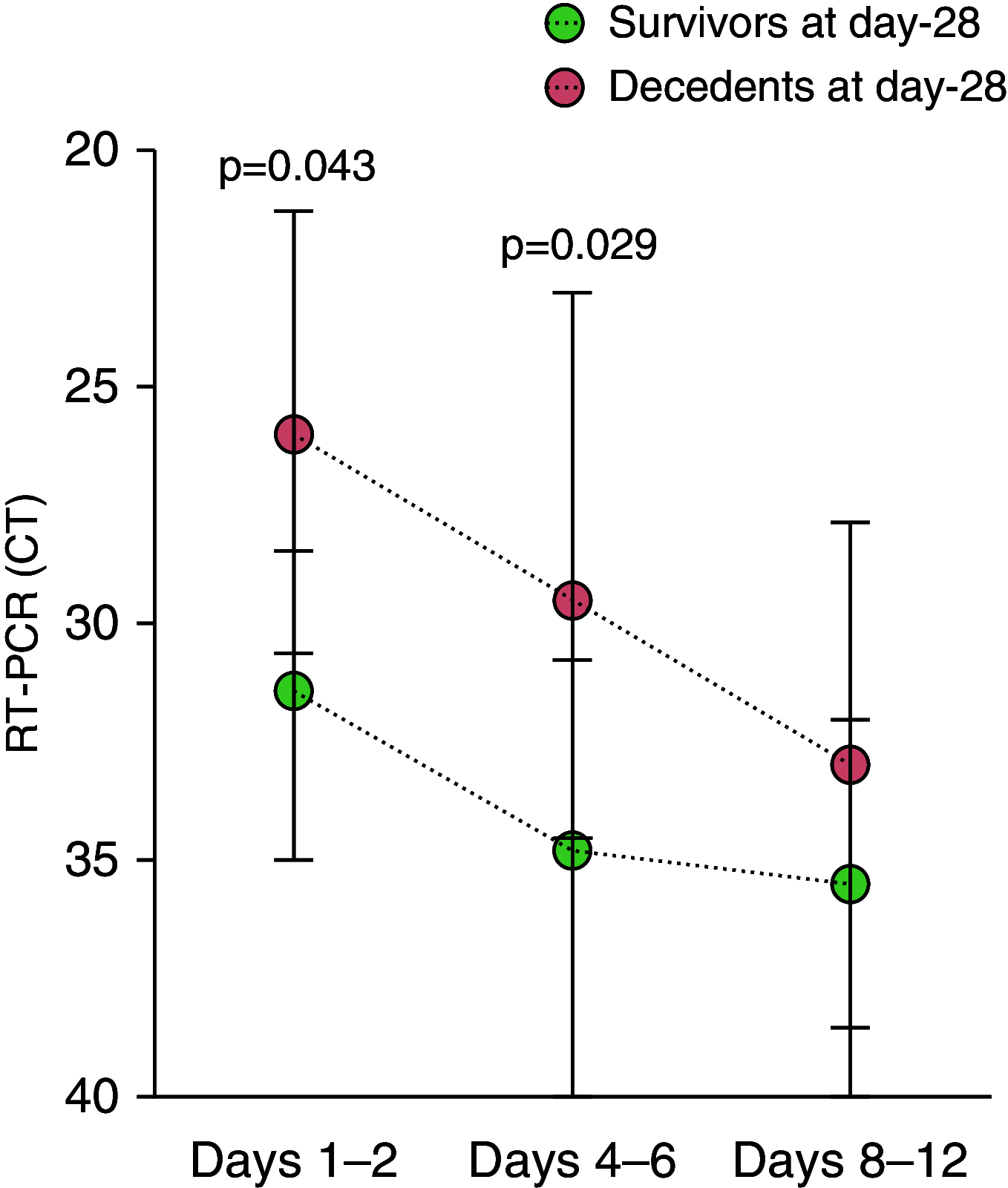
Rationale: Uncontrolled inflammatory innate response and impaired adaptive immune response are associated with clinical severity in patients with coronavirus disease (COVID-19).Objectives: To compare the immunopathology of COVID-19 acute respiratory distress syndrome (ARDS) with that of non-COVID-19 ARDS, and to identify biomarkers associated with mortality in patients with COVID-19 ARDS.Methods: Prospective observational monocenter study. Immunocompetent patients diagnosed with RT-PCR-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and ARDS admitted between March 8 and March 30, 2020, were included and compared with patients with non-COVID-19 ARDS. The primary clinical endpoint of the study was mortality at Day 28. Flow cytometry analyses and serum cytokine measurements were performed at Days 1-2 and 4-6 of ICU admission.Measurements and Main Results: As compared with patients with non-COVID-19 ARDS (n = 36), those with COVID-19 (n = 38) were not significantly different regarding age, sex, and Sequential Organ Failure Assessment and Simplified Acute Physiology Score II scores but exhibited a higher Day-28 mortality (34% vs. 11%, P = 0.030). Patients with COVID-19 showed profound and sustained T CD4+ (P = 0.002), CD8+ (P < 0.0001), and B (P < 0.0001) lymphopenia, higher HLA-DR expression on monocytes (P < 0.001) and higher serum concentrations of EGF (epithelial growth factor), GM-CSF, IL-10, CCL2/MCP-1, CCL3/MIP-1a, CXCL10/IP-10, CCL5/RANTES, and CCL20/MIP-3a. After adjusting on age and Sequential Organ Failure Assessment, serum CXCL10/IP-10 (P = 0.047) and GM-CSF (P = 0.050) were higher and nasopharyngeal RT-PCR cycle threshold values lower (P = 0.010) in patients with COVID-19 who were dead at Day 28.Conclusions: Profound global lymphopenia and a "chemokine signature" were observed in COVID-19 ARDS. Increased serum concentrations of CXCL10/IP-10 and GM-CSF, together with higher nasopharyngeal SARS-CoV-2 viral load, were associated with Day-28 mortality.
Keywords: ARDS; COVID-19; SARS-CoV-2; chemokines; cytokines.
Figures



Comment in
-
Biological Mechanisms of COVID-19 Acute Respiratory Distress Syndrome.Am J Respir Crit Care Med. 2020 Dec 1;202(11):1489-1491. doi: 10.1164/rccm.202009-3629ED. Am J Respir Crit Care Med. 2020. PMID: 32997945 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

