Telacebec (Q203)-containing intermittent oral regimens sterilized mice infected with Mycobacterium ulcerans after only 16 doses
- PMID: 32866170
- PMCID: PMC7494103
- DOI: 10.1371/journal.pntd.0007857
Telacebec (Q203)-containing intermittent oral regimens sterilized mice infected with Mycobacterium ulcerans after only 16 doses
Abstract
Buruli ulcer (BU), caused by Mycobacterium ulcerans, is currently treated with a daily combination of rifampin and either injectable streptomycin or oral clarithromycin. An intermittent oral regimen would facilitate treatment supervision. We first evaluated the bactericidal activity of newer antimicrobials against M. ulcerans using a BU animal model. The imidazopyridine amine telacebec (Q203) exhibited high bactericidal activity whereas tedizolid (an oxazolidinone closely related to linezolid), selamectin and ivermectin (two avermectine compounds) and the benzothiazinone PBTZ169 were not active. Consequently, telacebec was evaluated for its bactericidal and sterilizing activities in combined intermittent regimens. Telacebec given twice a week in combination with a long-half-life compound, either rifapentine or bedaquiline, sterilized mouse footpads in 8 weeks, i.e. after a total of only 16 doses, and prevented relapse during a period of 20 weeks after the end of treatment. These results are very promising for future intermittent oral regimens which would greatly simplify BU treatment in the field.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

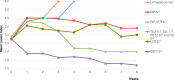
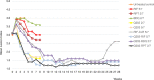
Similar articles
-
Shortening Buruli Ulcer Treatment with Combination Therapy Targeting the Respiratory Chain and Exploiting Mycobacterium ulcerans Gene Decay.Antimicrob Agents Chemother. 2019 Jun 24;63(7):e00426-19. doi: 10.1128/AAC.00426-19. Print 2019 Jul. Antimicrob Agents Chemother. 2019. PMID: 31036687 Free PMC article.
-
Sterilizing Activity of Fully Oral Intermittent Regimens against Mycobacterium Ulcerans Infection in Mice.PLoS Negl Trop Dis. 2016 Oct 18;10(10):e0005066. doi: 10.1371/journal.pntd.0005066. eCollection 2016 Oct. PLoS Negl Trop Dis. 2016. PMID: 27755552 Free PMC article.
-
Oxazolidinones Can Replace Clarithromycin in Combination with Rifampin in a Mouse Model of Buruli Ulcer.Antimicrob Agents Chemother. 2019 Feb 26;63(3):e02171-18. doi: 10.1128/AAC.02171-18. Print 2019 Mar. Antimicrob Agents Chemother. 2019. PMID: 30559131 Free PMC article.
-
Treating Mycobacterium ulcerans disease (Buruli ulcer): from surgery to antibiotics, is the pill mightier than the knife?Future Microbiol. 2011 Oct;6(10):1185-98. doi: 10.2217/fmb.11.101. Future Microbiol. 2011. PMID: 22004037 Free PMC article. Review.
-
Pharmacologic management of Mycobacterium ulcerans infection.Expert Rev Clin Pharmacol. 2020 Apr;13(4):391-401. doi: 10.1080/17512433.2020.1752663. Epub 2020 Apr 20. Expert Rev Clin Pharmacol. 2020. PMID: 32310683 Free PMC article. Review.
Cited by
-
Efficacy of PBTZ169 and pretomanid against Mycobacterium avium, Mycobacterium abscessus, Mycobacterium chelonae, and Mycobacterium fortuitum in BALB/c mice models.Front Cell Infect Microbiol. 2023 Mar 22;13:1115530. doi: 10.3389/fcimb.2023.1115530. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37077530 Free PMC article.
-
Unprecedented in vivo activity of telacebec against Mycobacterium leprae.PLoS Negl Trop Dis. 2025 May 8;19(5):e0013076. doi: 10.1371/journal.pntd.0013076. eCollection 2025 May. PLoS Negl Trop Dis. 2025. PMID: 40338896 Free PMC article.
-
Recent Advances in the Management Strategies for Buruli Ulcers.Pathogens. 2023 Aug 27;12(9):1088. doi: 10.3390/pathogens12091088. Pathogens. 2023. PMID: 37764896 Free PMC article. Review.
-
In vitro activity of SPR719 against Mycobacterium ulcerans, Mycobacterium marinum and Mycobacterium chimaera.PLoS Negl Trop Dis. 2021 Jul 26;15(7):e0009636. doi: 10.1371/journal.pntd.0009636. eCollection 2021 Jul. PLoS Negl Trop Dis. 2021. PMID: 34310615 Free PMC article.
-
Unlocking Opportunities for Mycobacterium leprae and Mycobacterium ulcerans.ACS Infect Dis. 2024 Feb 9;10(2):251-269. doi: 10.1021/acsinfecdis.3c00371. Epub 2024 Jan 31. ACS Infect Dis. 2024. PMID: 38295025 Free PMC article. Review.
References
-
- WHO. Treatment of Mycobacterium ulcerans disease (Buruli ulcer) Guidance for health workers. 2012. Available: https://apps.who.int/iris/bitstream/handle/10665/77771/9789241503402_eng...
-
- Phillips RO, Robert J, Abass KM, Thompson W, Sarfo FS, Wilson T, et al. Rifampicin and clarithromycin (extended release) versus rifampicin and streptomycin for limited Buruli ulcer lesions: a randomised, open-label, non-inferiority phase 3 trial. Lancet Lond Engl. 2020;395: 1259–1267. 10.1016/S0140-6736(20)30047-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

