Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study
- PMID: 32866433
- PMCID: PMC7455160
- DOI: 10.1016/S2468-1253(20)30271-5
Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study
Abstract
Background: Despite concerns that patients with liver transplants might be at increased risk of adverse outcomes from COVID-19 because of coexisting comorbidities and use of immunosuppressants, the effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection on this patient group remains unclear. We aimed to assess the clinical outcomes in these patients.
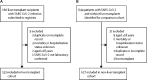
Methods: In this multicentre cohort study, we collected data on patients with laboratory-confirmed SARS-CoV-2 infection, who were older than 18 years, who had previously received a liver transplant, and for whom data had been submitted by clinicians to one of two international registries (COVID-Hep and SECURE-Cirrhosis) at the end of the patient's disease course. Patients without a known hospitalisation status or mortality outcome were excluded. For comparison, data from a contemporaneous cohort of consecutive patients with SARS-CoV-2 infection who had not received a liver transplant were collected from the electronic patient records of the Oxford University Hospitals National Health Service Foundation Trust. We compared the cohorts with regard to several outcomes (including death, hospitalisation, intensive care unit [ICU] admission, requirement for intensive care, and need for invasive ventilation). A propensity score-matched analysis was done to test for an association between liver transplant and death.
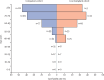
Findings: Between March 25 and June 26, 2020, data were collected for 151 adult liver transplant recipients from 18 countries (median age 60 years [IQR 47-66], 102 [68%] men, 49 [32%] women) and 627 patients who had not undergone liver transplantation (median age 73 years [44-84], 329 [52%] men, 298 [48%] women). The groups did not differ with regard to the proportion of patients hospitalised (124 [82%] patients in the liver transplant cohort vs 474 [76%] in the comparison cohort, p=0·106), or who required intensive care (47 [31%] vs 185 [30%], p=0·837). However, ICU admission (43 [28%] vs 52 [8%], p<0·0001) and invasive ventilation (30 [20%] vs 32 [5%], p<0·0001) were more frequent in the liver transplant cohort. 28 (19%) patients in the liver transplant cohort died, compared with 167 (27%) in the comparison cohort (p=0·046). In the propensity score-matched analysis (adjusting for age, sex, creatinine concentration, obesity, hypertension, diabetes, and ethnicity), liver transplantation did not significantly increase the risk of death in patients with SARS-CoV-2 infection (absolute risk difference 1·4% [95% CI -7·7 to 10·4]). Multivariable logistic regression analysis showed that age (odds ratio 1·06 [95% CI 1·01 to 1·11] per 1 year increase), serum creatinine concentration (1·57 [1·05 to 2·36] per 1 mg/dL increase), and non-liver cancer (18·30 [1·96 to 170·75]) were associated with death among liver transplant recipients.
Interpretation: Liver transplantation was not independently associated with death, whereas increased age and presence of comorbidities were. Factors other than transplantation should be preferentially considered in relation to physical distancing and provision of medical care for patients with liver transplants during the COVID-19 pandemic.
Funding: European Association for the Study of the Liver, US National Institutes of Health, UK National Institute for Health Research.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
SARS-CoV-2 infection in liver transplant recipients: collaboration in the time of COVID-19.Lancet Gastroenterol Hepatol. 2020 Nov;5(11):958-960. doi: 10.1016/S2468-1253(20)30293-4. Epub 2020 Sep 18. Lancet Gastroenterol Hepatol. 2020. PMID: 32956620 Free PMC article. No abstract available.
-
COVID-19 and liver transplantation: the jury is still out - Authors' reply.Lancet Gastroenterol Hepatol. 2021 Jan;6(1):11. doi: 10.1016/S2468-1253(20)30337-X. Epub 2020 Oct 30. Lancet Gastroenterol Hepatol. 2021. PMID: 33137285 Free PMC article. No abstract available.
-
COVID-19 and liver transplantation: the jury is still out.Lancet Gastroenterol Hepatol. 2021 Jan;6(1):10-11. doi: 10.1016/S2468-1253(20)30313-7. Epub 2020 Oct 30. Lancet Gastroenterol Hepatol. 2021. PMID: 33137286 Free PMC article. No abstract available.
References
-
- D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26:832–834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

