Dalbavancin exposure in vitro selects for dalbavancin-non-susceptible and vancomycin-intermediate strains of methicillin-resistant Staphylococcus aureus
- PMID: 32866650
- PMCID: PMC7914275
- DOI: 10.1016/j.cmi.2020.08.025
Dalbavancin exposure in vitro selects for dalbavancin-non-susceptible and vancomycin-intermediate strains of methicillin-resistant Staphylococcus aureus
Abstract
Objectives: Dalbavancin is a lipoglycopeptide active against methicillin-resistant Staphylococcus aureus (MRSA). Its long half-life (8.5-16 days) allows for once-weekly or single-dose treatments but could prolong the mutant selection window, promoting resistance and cross-resistance to related antimicrobials such as vancomycin. The objective of this study was to evaluate the capacity of post-distributional pharmacokinetic exposures of dalbavancin to select for resistance and cross-resistance in MRSA.
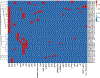
Methods: We simulated average, post-distributional exposures of single-dose (1500 mg) dalbavancin (fCmax 9.9 μg/mL, β-elimination t1/2 204 h) in an in vitro pharmacokinetic/pharmacodynamic (PK/PD) model for 28 days (672 h) against five MRSA strains and one methicillin-susceptible strain (MSSA). Samples were collected at least daily, and surviving colonies were enumerated and screened for resistance on drug-free and dalbavancin-supplemented medium respectively. Isolates from resistance screening plates were subjected to whole-genome sequencing (WGS) and susceptibly testing against dalbavancin, vancomycin, daptomycin, and six β-lactams with varying penicillin-binding protein (PBP) affinities.
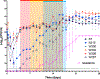
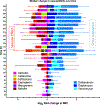
Results: Dalbavancin was bactericidal against most strains for days 1-4 before regrowth of less susceptible subpopulations occurred. Isolates with eight-fold increases in dalbavancin MIC were detected as early as day 4 but increased 64-128-fold in all models by day 28. Vancomycin and daptomycin MICs increased 4-16-fold, exceeding the susceptibly breakpoints for both antibiotics; β-lactam MICs generally decreased by two-to eight-fold, suggesting a dalbavancin-β-lactam seesaw effect, but increased by eight-fold or more in certain isolates. Resistant isolates carried mutations in a variety of genes, most commonly walKR, apt, stp1, and atl.
Conclusions: In our in vitro system, post-distributional dalbavancin exposures selected for stable mutants with reduced susceptibility to dalbavancin, vancomycin, and daptomycin, and generally increased susceptibility to β-lactams in all strains of MRSA tested. The clinical significance of these findings remains unclear, but created an opportunity to genotype a unique collection of dalbavancin-resistant strains for the first time. Mutations involved genes previously associated with vancomycin intermediate susceptibility and daptomycin non-susceptibility, most commonly walKR-associated genes.
Keywords: Cross-resistance; Daptomycin; Lipoglycopeptide; MRSA; PK/PD; Seesaw effect; VISA; walK; walR; β-lactam.
Copyright © 2020 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Simulated exposures of oritavancin in in vitro pharmacodynamic models select for methicillin-resistant Staphylococcus aureus with reduced susceptibility to oritavancin but minimal cross-resistance or seesaw effect with other antimicrobials.J Antimicrob Chemother. 2025 Apr 2;80(4):1108-1115. doi: 10.1093/jac/dkaf042. J Antimicrob Chemother. 2025. PMID: 39936452
-
Emergence of dalbavancin non-susceptible, vancomycin-intermediate Staphylococcus aureus (VISA) after treatment of MRSA central line-associated bloodstream infection with a dalbavancin- and vancomycin-containing regimen.Clin Microbiol Infect. 2018 Apr;24(4):429.e1-429.e5. doi: 10.1016/j.cmi.2017.07.028. Epub 2017 Aug 3. Clin Microbiol Infect. 2018. PMID: 28782651
-
Dalbavancin Alone and in Combination with Ceftaroline against Four Different Phenotypes of Staphylococcus aureus in a Simulated Pharmacodynamic/Pharmacokinetic Model.Antimicrob Agents Chemother. 2019 Mar 27;63(4):e01743-18. doi: 10.1128/AAC.01743-18. Print 2019 Apr. Antimicrob Agents Chemother. 2019. PMID: 30670436 Free PMC article.
-
New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin.Drugs. 2010 May 7;70(7):859-86. doi: 10.2165/11534440-000000000-00000. Drugs. 2010. PMID: 20426497 Review.
-
Acute Bacterial Skin and Skin-Structure Infections, efficacy of Dalbavancin: a systematic review and meta-analysis.Expert Rev Anti Infect Ther. 2022 Nov;20(11):1477-1489. doi: 10.1080/14787210.2021.1828865. Epub 2020 Oct 21. Expert Rev Anti Infect Ther. 2022. PMID: 32981375
Cited by
-
Mutation in the Two-Component System Regulator YycH Leads to Daptomycin Tolerance in Methicillin-Resistant Staphylococcus aureus upon Evolution with a Population Bottleneck.Microbiol Spectr. 2022 Aug 31;10(4):e0168722. doi: 10.1128/spectrum.01687-22. Epub 2022 Aug 1. Microbiol Spectr. 2022. PMID: 35913149 Free PMC article.
-
Investigational agents for the treatment of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia: progress in clinical trials.Expert Opin Investig Drugs. 2022 Mar;31(3):263-279. doi: 10.1080/13543784.2022.2040015. Epub 2022 Feb 14. Expert Opin Investig Drugs. 2022. PMID: 35129409 Free PMC article.
-
Targeting Dalbavancin Inoculum Effect: Adjunctive Single Dose of Daptomycin.Infect Dis Ther. 2023 Oct;12(10):2485-2494. doi: 10.1007/s40121-023-00875-1. Epub 2023 Oct 5. Infect Dis Ther. 2023. PMID: 37798469 Free PMC article.
-
Investigating the effect of an identified mutation within a critical site of PAS domain of WalK protein in a vancomycin-intermediate resistant Staphylococcus aureus by computational approaches.BMC Microbiol. 2021 Sep 2;21(1):240. doi: 10.1186/s12866-021-02298-9. BMC Microbiol. 2021. PMID: 34474665 Free PMC article.
-
High-throughput analysis of lipidomic phenotypes of methicillin-resistant Staphylococcus aureus by coupling in situ 96-well cultivation and HILIC-ion mobility-mass spectrometry.Anal Bioanal Chem. 2023 Oct;415(25):6191-6199. doi: 10.1007/s00216-023-04890-6. Epub 2023 Aug 3. Anal Bioanal Chem. 2023. PMID: 37535099 Free PMC article.
References
-
- Drlica K, Zhao X, Mutant selection window hypothesis updated, Clin. Infect. Dis. 44(5) (2007) 681–8. - PubMed
-
- Blondeau JM, New concepts in antimicrobial susceptibility testing: the mutant prevention concentration and mutant selection window approach, Vet Dermatol 20(5–6) (2009) 383–96. - PubMed
-
- Durata Therapeutics International, Dalbavancin for Injection. NDA 021–883. <https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/021883Orig1s000C...>, 2014. (accessed 5/21/2016.).
-
- Werth BJ, Jain R, Hahn A, Cummings L, Weaver T, Waalkes A, Sengupta D, Salipante SJ, Rakita RM, Butler-Wu SM, Emergence of dalbavancin non-susceptible, vancomycin-intermediate Staphylococcus aureus (VISA) after treatment of MRSA central line-associated bloodstream infection with a dalbavancin- and vancomycin-containing regimen, Clin Microbiol Infect (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

