Comparative evaluation of SARS-CoV-2 IgG assays in India
- PMID: 32866811
- PMCID: PMC7444492
- DOI: 10.1016/j.jcv.2020.104609
Comparative evaluation of SARS-CoV-2 IgG assays in India
Abstract
Introduction: IgG immunoassays have been developed and used widely for clinical samples and serosurveys for SARS-CoV2, with most detecting antibodies against the spike/receptor-binding-domain or nucleocapsid. Limited information is available on comparative evaluation of IgG immunoassays against a clinical reference standard, i.e., RT-PCR positivity with >20 days of illness. This study addresses the need for comparing clinical performance of IgG immunoassays with respect to this alternate reference standard.
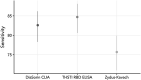
Methods: We compared the performance of three immunoassays, an in-house RBD assay, and two commercial assays, the Diasorin LIAISON SARS-CoV-2 S1/S1 IgG CLIA which detects antibodies against S1/S2 domains of the Spike protein and the Zydus Kavach assay based on inactivated virus using a well-characterized panel of sera. 379 sera and plasma samples from RTPCR positive individuals >20 days of illness in symptomatic or RT-PCR positivity in asymptomatic individuals and 184 samples collected prior to 2019 were used for assay evaluation.
Results: The sensitivity of the assays were 84.7 (95 %CI 80.6-88.1), 82.6 (95 %CI 78.3-86.2) and 75.7 (95 %CI 71.0-79.9) respectively for RBD, LIAISON and Kavach. Kavach and the in-house RBD ELISA showed a specificity of 99.5 % and 100 %, respectively. The RBD and LIAISON (S1/S2) assays showed high agreement (94.7 %; 95 %CI: 92.0, 96.6) and were able to correctly identify more positive sera/plasma than Kavach.
Conclusion: Independent comparisons support the evaluation of performance characteristics of immunoassays. All three assays are suitable for serosurveillance studies, but in low prevalence sites, estimation of exposure may require adjustment based on our findings.
Keywords: Immunoassay; LIAISON SARS-CoV-2 S1/S1 IgG; RBD IgG ELISA; SARS-CoV-2; Serology; Zydus Kavach.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
Figures
References
-
- SARS-CoV-2 diagnostic pipeline, FIND. https://www.finddx.org/covid-19/pipeline/ (accessed August 8, 2020).
-
- Strategy for COVID-19 testing in India. https://www.icmr.gov.in/pdf/covid/strategy/Testing_Strategy_v5_18052020.pdf (accessed August 7, 2020).
-
- SOP_Specimen_Collection_2019-nCoV.pdf. https://niv.co.in/SOP_Specimen_Collection_2019-nCoV.pdf (accessed August 7, 2020).
-
- Target Product Profile: enzyme Immunoassay (EIA) Antibody tests to help determine if people have antibodies to SARS-CoV-2, GOV.UK. https://www.gov.uk/government/publications/how-tests-and-testing-kits-fo... (accessed August 7, 2020).
-
- Public Health England Porton Down, Evaluation of sensitivity and specificity of four commercially available SARS-CoV-2 antibody immunoassays, Public Health England, Porton Down Nuffield Department of Medicine, University of Oxford Oxford University Hospitals NHS Foundation Trust, https://assets.publishing.service.gov.uk/government/uploads/system/uploa... (accessed August 7, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous