The mechanisms of action and use of botulinum neurotoxin type A in aesthetics: Key Clinical Postulates II
- PMID: 32866999
- PMCID: PMC7693297
- DOI: 10.1111/jocd.13702
The mechanisms of action and use of botulinum neurotoxin type A in aesthetics: Key Clinical Postulates II
Erratum in
-
Corrigendum.J Cosmet Dermatol. 2021 Jun;20(6):1954. doi: 10.1111/jocd.14108. Epub 2021 Apr 12. J Cosmet Dermatol. 2021. PMID: 33900684 Free PMC article. No abstract available.
Abstract
Background: The literature on botulinum neurotoxin type A (BoNT-A) is extensive, often contradictory, and confounded by a competitive market of products and research attempting to distinguish brand individuality.
Methods: A comprehensive review of literature on the principles of BoNT-A in aesthetics as well as clinical examples.
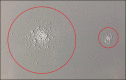
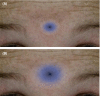
Results: In 2017, the Eight Key Clinical Postulates were formulated as a guide for the aesthetic practitioner in understanding BoNT-A pharmacodynamics and to compare different toxins. These are now updated to include (a) All type A toxins act identically; (b) The mathematical relationship between toxin and receptor is the basis of efficacy, and clinical efficacy is influenced by molecular potency and patient attributes including muscle mass, gender, age, and ethnicity; (c) Efficacy, onset, and duration are functions of "molecular potency" defined as the number of active 150 kDa molecules available for binding; (d) "Molecular potency" is difficult to objectively quantify for commercially available toxins; (e) Up to a point, increased molecular potency decreases time to onset and increases duration of effect, and the "Molecular Potency Quotient" is a construct for comparing molecular potency commercial cost; (f) The area of effect of a toxin injection is dependent upon molecular potency, diffusion (passive), and spread (active); (g) Differing reconstitution volumes; and (h) Increased number of injection sites can affect spread, onset, and duration of effect.
Conclusions: The principles of BoNT-A use in aesthetics are complex yet understandable as outlined in the framework of the updated Eight Key Clinical Postulates and serves as a useful tool for providing the most effective treatment and interpreting research on present and future toxin formulations.
Keywords: BoNT-A; botulinum neurotoxin type A; key clinical postulates; molecular potency; review.
© 2020 The Authors. Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
Croma Pharma, GmbH: Consultant, Research Grants; Galderma: Consultant, Research Grants; Ipsen: Consultant; Allergan: Advisory Board; Merz: Research Grants.
Figures
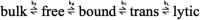



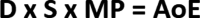
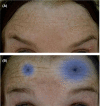
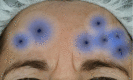
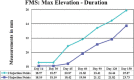
References
-
- Cohen BE, Bashey S, Wysong A. Literature review of cosmetic procedures in men: approaches and techniques are gender specific. Am J Clin Dermatol. 2017;18(1):87‐96. - PubMed
-
- drugs.com. drugs.com. Accessed January 3, 2020. drugs.com. https://www.drugs.com/international/botulinum-a-toxin.html
-
- Botulinum toxin A ‐ Allergan/Medytox ‐ AdisInsight. Available from https://adisinsight.springer.com/drugs/800021275. Accessed March 18, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

