Addition of Radiotherapy to Immunotherapy: Effects on Outcome of Different Subgroups Using a Propensity Score Matching
- PMID: 32867046
- PMCID: PMC7563550
- DOI: 10.3390/cancers12092429
Addition of Radiotherapy to Immunotherapy: Effects on Outcome of Different Subgroups Using a Propensity Score Matching
Abstract
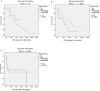
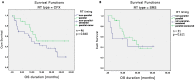
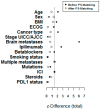
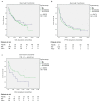
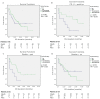
Immune checkpoint inhibition (ICI) has been established as successful modality in cancer treatment. Combination concepts are used to optimize treatment outcome, but may also induce higher toxicity rates than monotherapy. Several rationales support the combination of radiotherapy (RT) with ICI as radioimmunotherapy (RIT), but it is still unknown in which clinical situation RIT would be most beneficial. Therefore, we have conducted a retrospective matched-pair analysis of 201 patients with advanced-stage cancers and formed two groups treated with programmed cell death protein 1 (PD-1) inhibitors only (PD1i) or in combination with local RT (RIT) at our center between 2013 and 2017. We collected baseline characteristics, programmed death ligand 1 (PD-L1) status, mutational status, PD-1 inhibitor and RT treatment details, and side effects according to the Common Terminology Criteria for Adverse Events (CTCAE) v.5.0. Patients received pembrolizumab (n = 93) or nivolumab (n = 108), 153 with additional RT. For overall survival (OS) and progression-free survival (PFS), there was no significant difference between both groups. After propensity score matching (PSM), we analyzed 96 patients, 67 with additional and 29 without RT. We matched for different covariates that could have a possible influence on the treatment outcome. The RIT group displayed a trend towards a longer OS until the PD1i group reached a survival plateau. PD-L1-positive patients, smokers, patients with a BMI ≤ 25, and patients without malignant melanoma showed a longer OS when treated with RIT. Our data show that some subgroups may benefit more from RIT than others. Suitable biomarkers as well as the optimal timing and dosage must be established in order to achieve the best effect on cancer treatment outcome.
Keywords: PD-1/PD-L1; combination treatment; immune checkpoint inhibition; propensity score matching; radioimmunotherapy; radiotherapy; subgroups.
Conflict of interest statement
Consultant honoraria: BMS (C.B.). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Robert C., Ribas A., Schachter J., Arance A., Grob J.J., Mortier L., Daud A., Carlino M.S., McNeil C.M., Lotem M., et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1239–1251. doi: 10.1016/S1470-2045(19)30388-2. - DOI - PubMed
-
- Leighl N.B., Hellmann M.D., Hui R., Carcereny E., Felip E., Ahn M.-J., Eder J.P., Balmanoukian A.S., Aggarwal C., Horn L., et al. Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Respir. Med. 2019;7:347–357. doi: 10.1016/S2213-2600(18)30500-9. - DOI - PubMed
-
- Ferris R.L., Blumenschein G., Fayette J., Guigay J., Colevas A.D., Licitra L., Harringtong K.J., Kasper S., Vokes E.E., Even C., et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. doi: 10.1016/j.oraloncology.2018.04.008. - DOI - PMC - PubMed
-
- Sharma P., Callahan M.K., Bono P., Kim J.W., Spiliopoulou P., Calvo E., Pillai R.N., Ott P.A., De Braud F.G., Morse M., et al. Nivolumab monotherapy in metastatic urothelial carcinoma: Longer-term efficacy and safety results from the CheckMate 032 study. J. Clin. Oncol. 2018;36(Suppl. 6):414. doi: 10.1200/JCO.2018.36.6_suppl.414. - DOI
-
- Hodi F.S., Chiarion-Sileni V., Gonzalez R., Grob J.J., Rutkowski P., Cowey C.L., Lao C.D., Schadendorf D., Wagstaff J., Dummer R., et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–1492. doi: 10.1016/S1470-2045(18)30700-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

