Pharmacological Aspects of Over-the-Counter Opioid Drugs Misuse
- PMID: 32867117
- PMCID: PMC7504308
- DOI: 10.3390/molecules25173905
Pharmacological Aspects of Over-the-Counter Opioid Drugs Misuse
Abstract
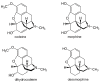
Several over-the-counter (OTC) drugs are known to be misused. Among them are opioids such as codeine, dihydrocodeine, and loperamide. This work elucidates their pharmacology, interactions, safety profiles, and how pharmacology is being manipulated to misuse these common medications, with the aim to expand on the subject outlined by the authors focusing on abuse prevention and prevalence rates. The reviewed literature was identified in several online databases through searches conducted with phrases created by combining the international non-proprietary names of the drugs with terms related to drug misuse. The results show that OTC opioids are misused as an alternative for illicit narcotics, or prescription-only opioids. The potency of codeine and loperamide is strongly dependent on the individual enzymatic activity of CYP2D6 and CYP3A4, as well as P-glycoprotein function. Codeine can also be utilized as a substrate for clandestine syntheses of more potent drugs of abuse, namely desomorphine ("Krokodil"), and morphine. The dangerous methods used to prepare these substances can result in poisoning from toxic chemicals and impurities originating from the synthesis procedure. OTC opioids are generally safe when consumed in accordance with medical guidelines. However, the intake of supratherapeutic amounts of these substances may reveal surprising traits of common medications.
Keywords: abuse; codeine; dihydrocodeine; loperamide; misuse; opioid drugs; over-the-counter drugs; pharmacology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- DZIENNIK USTAW RZECZYPOSPOLITEJ POLSKIEJ, Poz. 2189, ROZPORZĄDZENIE MINISTRA ZDROWIA z dnia 16 grudnia 2016 r. w sprawie wykazu substancji o działaniu psychoaktywnym oraz maksymalnego poziomu ich zawartości w produkcie leczniczym, stanowiącego ograniczenie w wydawaniu produktów leczniczych w ramach jednorazowej sprzedaży. [(accessed on 20 August 2020)]; Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20160002189/O/D20162189....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical