Supramolecular Chirality in Azobenzene-Containing Polymer System: Traditional Postpolymerization Self-Assembly Versus In Situ Supramolecular Self-Assembly Strategy
- PMID: 32867119
- PMCID: PMC7503415
- DOI: 10.3390/ijms21176186
Supramolecular Chirality in Azobenzene-Containing Polymer System: Traditional Postpolymerization Self-Assembly Versus In Situ Supramolecular Self-Assembly Strategy
Abstract
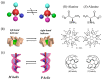
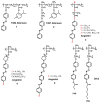
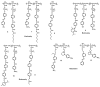
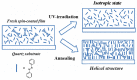
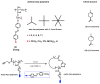
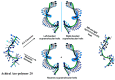
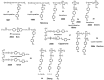
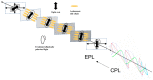
Recently, the design of novel supramolecular chiral materials has received a great deal of attention due to rapid developments in the fields of supramolecular chemistry and molecular self-assembly. Supramolecular chirality has been widely introduced to polymers containing photoresponsive azobenzene groups. On the one hand, supramolecular chiral structures of azobenzene-containing polymers (Azo-polymers) can be produced by nonsymmetric arrangement of Azo units through noncovalent interactions. On the other hand, the reversibility of the photoisomerization also allows for the control of the supramolecular organization of the Azo moieties within polymer structures. The construction of supramolecular chirality in Azo-polymeric self-assembled system is highly important for further developments in this field from both academic and practical points of view. The postpolymerization self-assembly strategy is one of the traditional strategies for mainly constructing supramolecular chirality in Azo-polymers. The in situ supramolecular self-assembly mediated by polymerization-induced self-assembly (PISA) is a facile one-pot approach for the construction of well-defined supramolecular chirality during polymerization process. In this review, we focus on a discussion of supramolecular chirality of Azo-polymer systems constructed by traditional postpolymerization self-assembly and PISA-mediated in situ supramolecular self-assembly. Furthermore, we will also summarize the basic concepts, seminal studies, recent trends, and perspectives in the constructions and applications of supramolecular chirality based on Azo-polymers with the hope to advance the development of supramolecular chirality in chemistry.
Keywords: Azo-containing polymers; in situ polymerization-induced self-assembly; postpolymerization self-assembly; supramolecular chirality.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

