Retroperitoneal Sarcomas: An Update on the Diagnostic Pathology Approach
- PMID: 32867125
- PMCID: PMC7555595
- DOI: 10.3390/diagnostics10090642
Retroperitoneal Sarcomas: An Update on the Diagnostic Pathology Approach
Abstract
Retroperitoneal sarcomas are a heterogenous group of rare tumors arising in the retroperitoneum. Retroperitoneal sarcomas comprise approximately 10% of all soft tissue sarcomas. Though any soft tissue sarcoma histologic types may arise in the retroperitoneal space, liposarcoma (especially well-differentiated and dedifferentiated types) and leiomyosarcoma do so most commonly. Retroperitoneal sarcomas are diagnostically challenging, owing to their diversity and morphological overlap with other tumors arising in the retroperitoneum. An accurate diagnosis is necessary for correct management and prognostication. Herein, we provide an update on the diagnostic approach to retroperitoneal sarcomas and review their key histologic findings and differential diagnoses.
Keywords: leiomyosarcoma; liposarcoma; pathology; retroperitoneal space; sarcoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

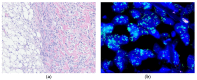
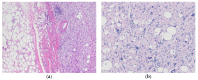


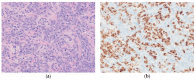
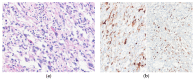






Similar articles
-
Updates in Pathology for Retroperitoneal Soft Tissue Sarcoma.Curr Oncol. 2022 Sep 7;29(9):6400-6418. doi: 10.3390/curroncol29090504. Curr Oncol. 2022. PMID: 36135073 Free PMC article. Review.
-
Retroperitoneal liposarcoma in older person - a rare case report.Aging Male. 2020 Dec;23(5):1509-1511. doi: 10.1080/13685538.2020.1813704. Epub 2020 Sep 11. Aging Male. 2020. PMID: 32912016
-
Importance of preoperative diagnosis for management of patients with suspected retroperitoneal sarcoma.ANZ J Surg. 2018 Apr;88(4):274-277. doi: 10.1111/ans.14125. Epub 2017 Aug 2. ANZ J Surg. 2018. PMID: 28768365 Review.
-
Retroperitoneal Liposarcoma: The Giant Type.J Med Cases. 2022 Oct;13(10):517-520. doi: 10.14740/jmc4014. Epub 2022 Oct 31. J Med Cases. 2022. PMID: 36407863 Free PMC article.
-
A huge retroperitoneal liposarcoma: case report.Eur J Gynaecol Oncol. 2012;33(3):318-20. Eur J Gynaecol Oncol. 2012. PMID: 22873110 Review.
Cited by
-
Practical Management of Adult Ultra-Rare Primary Retroperitoneal Soft Tissue Sarcoma: A Focus on Perivascular Epithelioid Tumours and Extraosseous Ewing Sarcoma.Curr Oncol. 2023 Jun 21;30(7):5953-5972. doi: 10.3390/curroncol30070445. Curr Oncol. 2023. PMID: 37504306 Free PMC article. Review.
-
A rare case of abdominal (lesser sac) myxoid liposarcoma: A case report.Int J Surg Case Rep. 2025 May;130:111275. doi: 10.1016/j.ijscr.2025.111275. Epub 2025 Apr 9. Int J Surg Case Rep. 2025. PMID: 40250185 Free PMC article.
-
Lipoleiomyosarcoma Presenting as Massive Retroperitoneal Mass: An Unusual Soft Tissue Tumor of Abdomen and its Review of Literature.Indian J Surg Oncol. 2024 Sep;15(Suppl 3):418-422. doi: 10.1007/s13193-024-01989-9. Epub 2024 Jul 2. Indian J Surg Oncol. 2024. PMID: 39328721
-
Synchronous Occurrence of Advanced Gastric Carcinoma with Retroperitoneal Liposarcoma: A Case Report.Am J Case Rep. 2022 Jan 8;23:e934586. doi: 10.12659/AJCR.934586. Am J Case Rep. 2022. PMID: 34996885 Free PMC article.
-
Retroperitoneal leiomyosarcoma invading the renal hilum: A case report and literature review.Radiol Case Rep. 2025 Aug 5;20(10):5300-5305. doi: 10.1016/j.radcr.2025.07.030. eCollection 2025 Oct. Radiol Case Rep. 2025. PMID: 40843294 Free PMC article.
References
-
- Fletcher C.D.M., Baldini E.H., Blay J.Y., Gronchi A., Lazar A.J., Messiou C., Pollock R.E., Singer S. Soft tissue tumors: Introduction. In: The WHO Classification of Tumours Editorial Board, editor. WHO Classification of Tumours. Soft Tissue and Bone Tumours. 5th ed. IARC Press; Lyon, France: 2020. pp. 1–12.
-
- Hornick J.L. Introduction: Tumor classification and immunohistochemistry. In: Hornick J.L., editor. Practical Soft Tissue Pathology: A Diagnostic Approach. 2nd ed. Elsevier; Philadelphia, PA, USA: 2019. pp. 1–6.
-
- The WHO Classification of Tumours Editorial Board . WHO Classification of Tumours. Soft Tissue and Bone Tumours. 5th ed. IARC Press; Lyon, France: 2020.
-
- Rosai J. Rosai and Ackerman’s Surgical Pathology. 10th ed. Elsevier; Philadelphia, PA, USA: 2011. pp. 2251–2258.
Publication types
LinkOut - more resources
Full Text Sources

