Ternary Cu(II) Complex with GHK Peptide and Cis-Urocanic Acid as a Potential Physiologically Functional Copper Chelate
- PMID: 32867146
- PMCID: PMC7503498
- DOI: 10.3390/ijms21176190
Ternary Cu(II) Complex with GHK Peptide and Cis-Urocanic Acid as a Potential Physiologically Functional Copper Chelate
Abstract
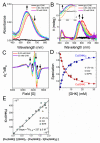
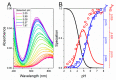
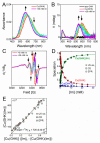
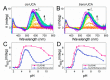
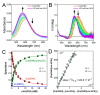
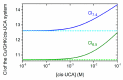
The tripeptide NH2-Gly-His-Lys-COOH (GHK), cis-urocanic acid (cis-UCA) and Cu(II) ions are physiological constituents of the human body and they co-occur (e.g., in the skin and the plasma). While GHK is known as Cu(II)-binding molecule, we found that urocanic acid also coordinates Cu(II) ions. Furthermore, both ligands create ternary Cu(II) complex being probably physiologically functional species. Regarding the natural concentrations of the studied molecules in some human tissues, together with the affinities reported here, we conclude that the ternary complex [GHK][Cu(II)][cis-urocanic acid] may be partly responsible for biological effects of GHK and urocanic acid described in the literature.
Keywords: copper; imidazole ligands; ternary complex.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Intermediate Cu(II)-Thiolate Species in the Reduction of Cu(II)GHK by Glutathione: A Handy Chelate for Biological Cu(II) Reduction.Inorg Chem. 2021 Dec 6;60(23):18048-18057. doi: 10.1021/acs.inorgchem.1c02669. Epub 2021 Nov 15. Inorg Chem. 2021. PMID: 34781677 Free PMC article.
-
X-ray and solution structures of Cu(II) GHK and Cu(II) DAHK complexes: influence on their redox properties.Chemistry. 2011 Aug 29;17(36):10151-60. doi: 10.1002/chem.201100751. Epub 2011 Jul 20. Chemistry. 2011. PMID: 21780203
-
Thermodynamic study of Cu2+ binding to the DAHK and GHK peptides by isothermal titration calorimetry (ITC) with the weaker competitor glycine.J Biol Inorg Chem. 2012 Jan;17(1):37-47. doi: 10.1007/s00775-011-0824-5. Epub 2011 Sep 4. J Biol Inorg Chem. 2012. PMID: 21898044
-
Are We Ready to Measure Skin Permeation of Modern Antiaging GHK-Cu Tripeptide Encapsulated in Liposomes?Molecules. 2025 Jan 1;30(1):136. doi: 10.3390/molecules30010136. Molecules. 2025. PMID: 39795193 Free PMC article. Review.
-
Studies to determine the immunomodulating effects of cis-urocanic acid.Methods. 2002 Sep;28(1):63-70. doi: 10.1016/s1046-2023(02)00210-4. Methods. 2002. PMID: 12231189 Review.
Cited by
-
Ergothioneine in a peptide: Substitution of histidine with 2-thiohistidine in bioactive peptides.J Pept Sci. 2021 Oct;27(10):e3339. doi: 10.1002/psc.3339. Epub 2021 May 18. J Pept Sci. 2021. PMID: 34008255 Free PMC article.
-
Intermediate Cu(II)-Thiolate Species in the Reduction of Cu(II)GHK by Glutathione: A Handy Chelate for Biological Cu(II) Reduction.Inorg Chem. 2021 Dec 6;60(23):18048-18057. doi: 10.1021/acs.inorgchem.1c02669. Epub 2021 Nov 15. Inorg Chem. 2021. PMID: 34781677 Free PMC article.
-
Reactive Cu2+-peptide intermediates revealed by kinetic studies gain relevance by matching time windows in copper metallomics.Metallomics. 2023 Feb 16;15(2):mfad007. doi: 10.1093/mtomcs/mfad007. Metallomics. 2023. PMID: 36787891 Free PMC article.
-
Essential Role of Histidine for Rapid Copper(II)-Mediated Disassembly of Neurokinin B Amyloid.Biomolecules. 2022 Oct 28;12(11):1585. doi: 10.3390/biom12111585. Biomolecules. 2022. PMID: 36358935 Free PMC article.
-
Chemistry towards Biology.Int J Mol Sci. 2023 Feb 16;24(4):3998. doi: 10.3390/ijms24043998. Int J Mol Sci. 2023. PMID: 36835407 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

