Improving Tumor Retention of Effector Cells in Adoptive Cell Transfer Therapies by Magnetic Targeting
- PMID: 32867162
- PMCID: PMC7557387
- DOI: 10.3390/pharmaceutics12090812
Improving Tumor Retention of Effector Cells in Adoptive Cell Transfer Therapies by Magnetic Targeting
Abstract
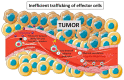
Adoptive cell transfer therapy is a promising anti-tumor immunotherapy in which effector immune cells are transferred to patients to treat tumors. However, one of its main limitations is the inefficient trafficking of inoculated effector cells to the tumor site and the small percentage of effector cells that remain activated when reaching the tumor. Multiple strategies have been attempted to improve the entry of effector cells into the tumor environment, often based on tumor types. It would be, however, interesting to develop a more general approach, to improve and facilitate the migration of specific activated effector lymphoid cells to any tumor type. We and others have recently demonstrated the potential for adoptive cell transfer therapy of the combined use of magnetic nanoparticle-loaded lymphoid effector cells together with the application of an external magnetic field to promote the accumulation and retention of lymphoid cells in specific body locations. The aim of this review is to summarize and highlight the recent findings in the field of magnetic accumulation and retention of effector cells in tumors after adoptive transfer, and to discuss the possibility of using this approach for tumor targeting with chimeric antigen receptor (CAR) T-cells.
Keywords: adoptive cell transfer therapy; cancer immunotherapy; chimeric antigen receptor (CAR) T-cells; magnetic targeting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Magnetic targeting of adoptively transferred tumour-specific nanoparticle-loaded CD8+ T cells does not improve their tumour infiltration in a mouse model of cancer but promotes the retention of these cells in tumour-draining lymph nodes.J Nanobiotechnology. 2019 Aug 6;17(1):87. doi: 10.1186/s12951-019-0520-0. J Nanobiotechnology. 2019. PMID: 31387604 Free PMC article.
-
Engineered CAR-Macrophages as Adoptive Immunotherapies for Solid Tumors.Front Immunol. 2021 Nov 24;12:783305. doi: 10.3389/fimmu.2021.783305. eCollection 2021. Front Immunol. 2021. PMID: 34899748 Free PMC article. Review.
-
Magnetic Nanoparticles Attached to the NK Cell Surface for Tumor Targeting in Adoptive Transfer Therapies Does Not Affect Cellular Effector Functions.Front Immunol. 2019 Aug 30;10:2073. doi: 10.3389/fimmu.2019.02073. eCollection 2019. Front Immunol. 2019. PMID: 31543880 Free PMC article.
-
Engineering strategies for broad application of TCR-T- and CAR-T-cell therapies.Int Immunol. 2021 Oct 29;33(11):551-562. doi: 10.1093/intimm/dxab052. Int Immunol. 2021. PMID: 34374779 Review.
-
Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines.Oncoimmunology. 2018 Jan 9;7(3):e1395997. doi: 10.1080/2162402X.2017.1395997. eCollection 2018. Oncoimmunology. 2018. PMID: 29399394 Free PMC article.
Cited by
-
Effects of Cationic Dendrimers and Their Complexes with microRNAs on Immunocompetent Cells.Pharmaceutics. 2022 Dec 31;15(1):148. doi: 10.3390/pharmaceutics15010148. Pharmaceutics. 2022. PMID: 36678776 Free PMC article.
-
Insight into lipid-based nanoplatform-mediated drug and gene delivery in neuro-oncology and their clinical prospects.Front Oncol. 2023 Jul 6;13:1168454. doi: 10.3389/fonc.2023.1168454. eCollection 2023. Front Oncol. 2023. PMID: 37483515 Free PMC article. Review.
-
Helios as a Potential Biomarker in Systemic Lupus Erythematosus and New Therapies Based on Immunosuppressive Cells.Int J Mol Sci. 2023 Dec 29;25(1):452. doi: 10.3390/ijms25010452. Int J Mol Sci. 2023. PMID: 38203623 Free PMC article. Review.
-
Understanding MNPs Behaviour in Response to AMF in Biological Milieus and the Effects at the Cellular Level: Implications for a Rational Design That Drives Magnetic Hyperthermia Therapy toward Clinical Implementation.Cancers (Basel). 2021 Sep 12;13(18):4583. doi: 10.3390/cancers13184583. Cancers (Basel). 2021. PMID: 34572810 Free PMC article. Review.
-
Progress and Viewpoints of Multifunctional Composite Nanomaterials for Glioblastoma Theranostics.Pharmaceutics. 2022 Feb 21;14(2):456. doi: 10.3390/pharmaceutics14020456. Pharmaceutics. 2022. PMID: 35214188 Free PMC article. Review.

