Aggrecan, the Primary Weight-Bearing Cartilage Proteoglycan, Has Context-Dependent, Cell-Directive Properties in Embryonic Development and Neurogenesis: Aggrecan Glycan Side Chain Modifications Convey Interactive Biodiversity
- PMID: 32867198
- PMCID: PMC7564073
- DOI: 10.3390/biom10091244
Aggrecan, the Primary Weight-Bearing Cartilage Proteoglycan, Has Context-Dependent, Cell-Directive Properties in Embryonic Development and Neurogenesis: Aggrecan Glycan Side Chain Modifications Convey Interactive Biodiversity
Abstract
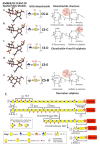
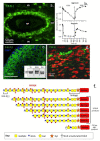
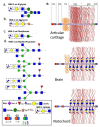
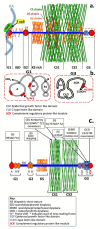
This review examines aggrecan's roles in developmental embryonic tissues, in tissues undergoing morphogenetic transition and in mature weight-bearing tissues. Aggrecan is a remarkably versatile and capable proteoglycan (PG) with diverse tissue context-dependent functional attributes beyond its established role as a weight-bearing PG. The aggrecan core protein provides a template which can be variably decorated with a number of glycosaminoglycan (GAG) side chains including keratan sulphate (KS), human natural killer trisaccharide (HNK-1) and chondroitin sulphate (CS). These convey unique tissue-specific functional properties in water imbibition, space-filling, matrix stabilisation or embryonic cellular regulation. Aggrecan also interacts with morphogens and growth factors directing tissue morphogenesis, remodelling and metaplasia. HNK-1 aggrecan glycoforms direct neural crest cell migration in embryonic development and is neuroprotective in perineuronal nets in the brain. The ability of the aggrecan core protein to assemble CS and KS chains at high density equips cartilage aggrecan with its well-known water-imbibing and weight-bearing properties. The importance of specific arrangements of GAG chains on aggrecan in all its forms is also a primary morphogenetic functional determinant providing aggrecan with unique tissue context dependent regulatory properties. The versatility displayed by aggrecan in biodiverse contexts is a function of its GAG side chains.
Keywords: HNK-1 trisaccharide; aggrecan; cellular regulation; extracellular matrix; glycosaminoglycan; tissue morphogenesis.
Conflict of interest statement
The authors declare no conflict of interest and have no financial disclosures to make.
Figures


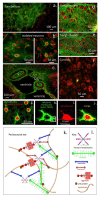
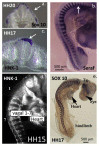
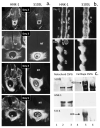
Similar articles
-
Isolation and characterization of chondroitin sulfate proteoglycans from embryonic quail that influence neural crest cell behavior.Dev Biol. 1997 Dec 1;192(1):108-24. doi: 10.1006/dbio.1997.8731. Dev Biol. 1997. PMID: 9405101
-
Inhibitory effects of PG-H/aggrecan and PG-M/versican on avian neural crest cell migration.FASEB J. 1996 Feb;10(2):293-301. doi: 10.1096/fasebj.10.2.8641562. FASEB J. 1996. PMID: 8641562
-
Developmental expression of the HNK-1 carbohydrate epitope on aggrecan during chondrogenesis.Dev Dyn. 2003 Jan;226(1):42-50. doi: 10.1002/dvdy.10214. Dev Dyn. 2003. PMID: 12508223
-
Keratan sulfate (KS)-proteoglycans and neuronal regulation in health and disease: the importance of KS-glycodynamics and interactive capability with neuroregulatory ligands.J Neurochem. 2019 Apr;149(2):170-194. doi: 10.1111/jnc.14652. Epub 2019 Jan 27. J Neurochem. 2019. PMID: 30578672 Review.
-
The structure, function and turnover of aggrecan, the large aggregating proteoglycan from cartilage.Eur J Clin Chem Clin Biochem. 1994 Apr;32(4):249-57. Eur J Clin Chem Clin Biochem. 1994. PMID: 8038265 Review.
Cited by
-
Therapeutic Potential of Olfactory Ensheathing Cells and Adipose-Derived Stem Cells in Osteoarthritis: Insights from Preclinical Studies.Cells. 2024 Jul 25;13(15):1250. doi: 10.3390/cells13151250. Cells. 2024. PMID: 39120281 Free PMC article.
-
Preface for the Special Issue on the Exploration of the Multifaceted Roles of Glycosaminoglycans: GAGs.Biomolecules. 2021 Nov 4;11(11):1630. doi: 10.3390/biom11111630. Biomolecules. 2021. PMID: 34827628 Free PMC article.
-
Recent Advances in the Treatment of Spinal Cord Injury.Arch Bone Jt Surg. 2024;12(6):380-399. doi: 10.22038/ABJS.2023.73944.3424. Arch Bone Jt Surg. 2024. PMID: 38919744 Free PMC article. Review.
-
Inhibiting synovial inflammation and promoting cartilage repair in rheumatoid arthritis using a matrix metalloproteinase-binding hydrogel.Mater Today Bio. 2025 Apr 23;32:101792. doi: 10.1016/j.mtbio.2025.101792. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40343163 Free PMC article.
-
Development of the mechanoresponsive pericellular matrix of chondrons.Sci Adv. 2025 May 2;11(18):eado6644. doi: 10.1126/sciadv.ado6644. Epub 2025 May 2. Sci Adv. 2025. PMID: 40315311 Free PMC article.
References
-
- Hardingham T.E., Fosang A.J., Dudhia J. The structure, function and turnover of aggrecan, the large aggregating proteoglycan from cartilage. Eur. J. Clin. Chem. Clin. Biochem. 1994;32:249–257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

